Indexed in: ESCI, Scopus, PubMed,
PubMed Central, CAS, DOAJ, KCI
PubMed Central, CAS, DOAJ, KCI
FREE article processing charge

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 36(3); 2019 > Article
-
Review article
Prepectoral breast reconstruction -
Sung-Eun Kim

-
Yeungnam University Journal of Medicine 2019;36(3):201-207.
DOI: https://doi.org/10.12701/yujm.2019.00283
Published online: August 26, 2019
Department of Plastic and Reconstructive Surgery, Catholic University of Daegu School of Medicine, Daegu, Korea
- Corresponding author: Sung-Eun Kim, Department of Plastic and Reconstructive Surgery, Catholic University of Daegu School of Medicine, 33, Duryugongwon-ro 17-gil, Nam-gu, Daegu 42472, Korea Tel: +82-53-650-4578, Fax: +82-53-650-4584, Email: fdghfg26@cu.ac.kr
• Received: June 28, 2019 • Revised: August 12, 2019 • Accepted: August 14, 2019
Copyright © 2019 Yeungnam University College of Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 9,354 Views
- 127 Download
- 17 Crossref
Abstract
- Implant-based breast reconstruction is the most commonly used reconstruction technique after mastectomy. This is because skin-sparing mastectomy has become possible with advancements in oncology. In addition, the development of breast implants and the advent of acellular dermal matrices have reduced postoperative complications and resulted in superior cosmetic results. The most frequently performed surgical breast reconstruction procedure for the past 20 years was the insertion of an implant under the pectoralis major muscle by means of the dual plane approach. However, some patients suffered from pain and animation deformity caused by muscle manipulation. Recently, a prepectoral approach has been used to solve the above problems in select patients, and the results are similar to subpectoral results. However, this technique is not always chosen due to the number of considerations for successful surgery. In this article, we will discuss the emergence of prepectoral breast reconstruction, indications and contraindications, surgical procedures, and outcomes.
- Breast reconstruction is increasing every year alongside increases in breast cancer. According to American Society of Plastic Surgeons statistical report, the numbers of breast reconstructions in the United States in 2000 and 2018 were 78,832 and 101,657, respectively [1]. The most frequently used reconstructive method is an implant-based reconstruction. In 2018 in the United States, 83,216 implant-based reconstructions were performed, while 18,441 autologous tissue reconstructions were done [1]. Prosthetic breast reconstruction is the number one procedure because advancements in implants and improvements in mastectomy techniques have resulted in better aesthetic outcomes. Recently, an issue has arisen regarding which plane implants should be placed in. In other words, a reverse shift from submuscular to prepectoral placement has occurred.
Introduction
- The ideal location for implants was once thought to be under the skin where the original breast tissue was, above the pectoralis major muscle. Silicone or saline implants were inserted subcutaneously until the introduction of the tissue expander. This approach was simple and able to preserve muscles, but typically, the mastectomy flap was too thin, and the subcutaneous tissue was deficient, resulting in many complications associated with this method. Implants that became exposed through the skin increased the risk of infection of the implants. Ultimately, removal of the implants occurred frequently, and capsular contracture, in particular, was a common complication [2]. These led to a submuscular approach where the implants were completely covered by the pectoralis major and serratus anterior muscles using a prepectoral approach. Gruber et al. reported a comparison of submuscular and subcutaneous techniques for breast reconstruction following mastectomy [3] and concluded that submuscular implants are clearly superior to subcutaneous ones and that the subserratus techniques provided the lowest incidence of capsular contracture. Augmentation case studies have also concluded that the subpectoral approach is superior, especially in regard to capsular contracture [4,5].
- However, the full muscle coverage technique has some problems. It could not expand the lower pole and natural ptotic breasts. In addition, implants were not covered by only the pectoralis major muscle, and the recruitment of other muscle flaps, such as the serratus muscle or the rectus abdominis sheath, was needed. To solve these problems, the partial muscle coverage, or dual plane, technique was introduced. This technique enabled expansion of the lower pole, but the pectoralis major muscle was not fixed to the chest wall, so it often migrated superiorly, resulting in so-called window shading [6–8].
- These problems were addressed by the advent of acellular dermal matrices (ADMs). An ADM is a biotechnologically designed human tissue of bovine or porcine origin that has served numerous purposes across surgical subsectors. Tissue processing removes cellular antigens that can generate an immunological response while maintaining a structural matrix that promotes angiogenesis and tissue regeneration. In 1995, for the first time, ADM was used to treat full-thickness burns [9]. Then, in 2006, Salzberg published his experience using ADM in immediate breast reconstruction, and ADM became an important ingredient in breast reconstruction [10].
- Afterward, the dual plane approach of ADM and covering the implant with the pectoralis major muscle was commonly performed. In this procedure, the ADM was sutured to the inferior margin of the pectoralis major muscle, which enabled not only expansion of the lower pole but also a decreased incidence of window shading. In addition, the ADM defines the lateral inframammary fold and supports the inferolateral portion, resulting to minimize migration of the implant caused by muscle contraction.
- However, there still remain problems of animation deformity due to muscle contraction, as well as pain caused by the dissection of the muscle. Ultimately, plastic surgeons were reminded of the idea of inserting an implant in the site of the original breast tissue without operating again on the muscle. Notably, capsular contracture, which was the main complication of previous subcutaneous breast reconstruction procedures, has been reduced with the use of ADMs. Kim et al. reported that the levels of myofibroblasts were significantly lower in ADM capsules than in submuscular capsules [11]. Now, with advances in oncology such as the skin-sparing mastectomy with optimal skin flap, improved implants, and ADMs, prepectoral breast reconstruction has become feasible.
History
- Prepectoral breast reconstruction has obvious advantages. In addition to the surgical technique being simple and less invasive, the operation time is short, and the muscle is left intact, reducing bleeding, pain, and recovery time after surgery. The biggest advantage is that it significantly reduces the occurrence of animation deformities. Because the contraction of the muscle does not affect the implant, there is less implant migration. Despite these advantages, the use of this method is limited because the appropriateness of the mastectomy skin flap after oncologic resection determines the viability of the operation. In other words, good vascularity and sufficient subcutaneous fat tissue should remain (Table 1) [12]. If the skin flap is too thin, it can cause rippling and palpability problems. If the vascularity is poor, skin necrosis, infection, and other complications may occur. Therefore, for successful surgery, it is important to select appropriate patients through close cooperation between the breast surgeon and the plastic surgeon.
- Prepectoral breast reconstruction is especially recommended for athletes, who require extensive use of the pectoralis major muscle, or for those whose shoulder function should be preserved. However, this lifestyle alone cannot determine the operation. There are two major factors in play, one of which is adequate vascularization of the mastectomy skin flap, which can be assessed before and during surgery, and the other is the oncologic consideration.
- Patient factors that may affect the vascularity of the skin flap before surgery should be considered (Table 2) [13,14]. Poorly controlled blood glucose, obesity, and recent smokers are contraindications. Large breasts require large implants, resulting in a decrease of the perfusion of skin, so they are a contraindication. In addition, immunocompromised patients are at increased risk in general and are not suitable for this procedure. Another important factor is the irradiated status of the breast. Preoperative radiation therapy affects wound healing and increases rates of infection, skin necrosis, capsular contracture, and more. Instead of prepectoral breast reconstruction, it is recommended that these patients undergo autologous tissue reconstruction [15]. Although there are few studies related to prepectoral reconstruction and postmastectomy radiation therapy (PMRT), there are arguments that the prepectoral approach may be a more appropriate choice for patients receiving adjuvant radiation therapy than the dual plane approach. Sigalove et al. studied 33 patients who underwent 52 breast reconstructions via the prepectoral approach and the short-term outcomes. They concluded that an immediate implant-based prepectoral breast reconstruction followed by PMRT appeared to be well tolerated, with no excess risk of adverse outcomes [16]. According to Sinnott et al., patients undergoing submuscular breast reconstruction who received PMRT had a capsular contracture rate three times greater, with more severe contractures (Baker grade III or IV) than did patients receiving PMRT who underwent prepectoral breast reconstruction [17]. Sbitany et al. studied 26 breasts that underwent immediate prepectoral reconstruction and 31 breasts that underwent immediate submuscular/dual plane reconstruction in the setting of PMRT and found no significant differences in complication rates between the two reconstructive groups [18].
- It is important to evaluate the condition of the skin flap intraoperatively, and there is a method for clinically or objectively evaluating whether the perfusion of a mastectomy skin flap is good or bad, which will be discussed in detail in the surgical technique section.
- Another concern with prepectoral breast reconstruction is the problem associated with the detection of cancer recurrence. If the tumor is located close to the pectoralis muscle, with a subpectoral implant placement, a recurring tumor can be detected by palpation. However, with a prepectoral implant placement, tumor recurrence may be detected later. Therefore, the location of the tumor should be taken into consideration during prepectoral reconstruction.
Patient selection
- 1. Assessment of mastectomy skin flap
- Clinical examination is important for evaluating the vascularity of skin flaps (Fig. 1), and bleeding on the incision edge should be seen. Any subcutaneous fat should also be preserved because if there is only dermis and no fat, blood flow will be inadequate. In those cases, it is necessary to consider a dual plane placement or staged reconstruction rather than an immediate prepectoral placement. However, skin thinning is not necessarily contraindicated. Even with thin skin, if the subdermal plexus is preserved, prepectoral breast reconstruction is possible.
- Another method of assessing tissue perfusion is the use of indocyanine green angiography. This can evaluate the blood flow of arterial and venous vasculature in real time and helps to confirm the clinical examination. It is helpful to predict the viability of the skin flap, especially if the skin is expanded when the implant is placed, and it allows the surgeon to ensure that any skin flap expected to become necrotizing is removed.
- 2. Choice of implant and acellular dermal matrix
- The implant in a prepectoral reconstruction should be carefully selected to prevent rippling. To do so, the selected implant must have a base width dimension that is correct for the pocket of the ADM or skin flap. The cohesiveness of the implant is determined by the thickness of the skin [19]. The thinner the flap, the better it is to choose a highly cohesive gel implant. Less cohesive gel implants are more prone to wrinkling but could be selected for better projection because there is more collapse at the upper pole and descent to the lower pole. However, whether round or smooth can be selected dependent on the operator’s preference and the patient’s desire.
- With two-stage surgery, it is important to underfill the tissue expander to make the tight pocket in the second stage. The height of the expander is chosen depending on whether the implant is anatomic or round. Typically, short to medium height expanders are used in the first stage for planned round implants, and full height expanders are used for planned anatomical implants.
- The ADM is typically used in prepectoral breast reconstruction, but it is not necessarily used [20]. It has been reported that there was no difference in complication rates between groups where an ADM was used and groups where it was not, but it is clear that the incidence of capsular contracture is significantly lower in the ADM group [21–26]. The selected ADM is usually 2–3 mm thick. If a perforated ADM is used, fluid can flow in both directions, minimizing sticking between the ADM and the skin flap. In addition, the perforations create an adhesive area between the ADM and the skin flap, thereby promoting incorporation [27].
- 3. Acellular dermal matrix coverage
- There are various methods for covering an implant with an ADM [28], and there is some controversy whether it is better to use 1 large ADM sheet or to sew 2 or more sheets together. There is also controversy as to whether it is better to cover the implant only anteriorly or both anteriorly and posteriorly. The latter idea of total ADM coverage of the device has been assessed to demonstrate the lower incidence of capsular contracture [22,29]. Because ADMs are shaped in flat sheets and there are various sizes, there are more ways to cover the three-dimensional shape of implants. For example, Braxon (DECO med s.r.l., Marcon, Venice, Italy) is a preshaped, porcine, non-cross-linked ADM that can be wrapped around an implant.
- There are two main ways to place an ADM in the prepectoral space, according to US Food and Drug Administration (FDA) labeling. The FDA requires that an ADM for breast reconstruction is used for tissue support. Therefore, with the on-label method, a trimmed ADM is inserted into the prepectoral space, and after insertion of the implant, the edges of the ADM are sutured to the inframammary fold along the chest wall, leaving a 3–4 cm cuff along the pectoralis major muscle. With this method, an inferior “gutter” is created to support the lower pole. In contrast, with the off-label technique, the ADM supports the implant. The implant is wrapped with the ADM on the back table, making a peripheral “cuff,” and then the device with the ADM is inserted in the prepectoral space. In this method, the implant is either partially (Fig. 2) or completely (Fig. 3) wrapped with ADM. Whichever option the operator chooses, it is important for a permanent implant to align the ADM to the shape of the implant without laxity. For an expander, it is possible to make an ADM pocket with a little laxity. After insertion of the device into the mastectomy pocket, the ADM is fixated to the underlying pectoralis major muscle circumferentially using absorbable sutures. In cases where a tissue expander is used, it is fixed to the pectoralis to anchor the expander tabs.
Surgical technique
- There is a lack of long-term outcomes for prepectoral breast reconstruction. However, several authors have reported on the safety, functional and aesthetic outcomes, and complications. Sigalove et al. published preliminary results from over 350 prepectoral breast reconstructions using ADMs [30]. Complications such as infection, seroma, and flap necrosis occurred at rates of less than 5%, and there were no capsular contractures. Patients with prior radiation therapy had higher rates of complications (5 out of 10). In contrast, patients with PMRT had no complications. Zhu et al. reported on comparative studies of prepectoral and total muscle coverage breast reconstruction [31] where they demonstrated similar morbidity rates with regard to infection, superficial skin necrosis, and seroma in both groups but decreased rates of capsular contracture in the prepectoral group. Bernini et al. examined the surgical and aesthetic results of 34 subpectoral and 39 subcutaneous techniques using titanium-coated polypropylene mesh [32]. Although there were no significant differences between the groups, the subcutaneous group had an implant failure rate of 5.1%, while the subpectoral group had a 0% failure rate. The subcutaneous group also had significantly better aesthetic outcomes.
- Schaeffer et al. reported early functional outcomes following prepectoral breast reconstruction in comparison with subpectoral breast reconstruction [33]. They showed that the prepectoral groups had significantly lower inpatient pain scores. In addition, the range of shoulder motion in the prepectoral group had fully returned in half the number days as in the subpectoral group.
- Rippling and wrinkling are commonly seen in the setting of prepectoral reconstruction. There is also a clear step-off between the chest wall and the prepectoral implant. Although there are very few studies about the effect of autologous fat grafting on patient reported outcomes following prepectoral breast reconstruction, many authors have argued that the primary means for correcting these deformities is autologous fat grafting [13,30,34,35]. Advances in fat grafting techniques have made fat grafting a routine procedure in breast surgery [30]. In fact, in 2018 in the United States, nearly 30% of all breast reconstruction cases utilized autologous fat grafts [1]. This allows the soft tissue volume between the implant and the mastectomy flap to be supported and augmented.
- The cost of ADM is an issue in the setting of prepectoral reconstruction. Two or 4 times the size of ADM that is used for subpectoral breast reconstruction is needed for this procedure, which will certainly result in incurring additional costs in a range of $5,000 to $20,000 per breast. However, some authors have claimed that prepectoral breast reconstruction is economically advantageous, but more studies should be performed [25,36]. For cost-savings, other authors have noted the use of alternative materials such as Vicryl mesh [21], porcine mesh [22], or titanium-coated polypropylene mesh [32]. All have demonstrated success in the prepectoral setting and may have cost-saving benefits.
Outcomes
- Prepectoral breast reconstruction is a simple muscle-sparing technique that reduces pain and recovery time after surgery. Above all, the occurrence rate of animation deformities can be reduced. In addition, aesthetically superior outcomes have been demonstrated. A successful prepectoral approach is possible with appropriate patient selection, availability of ADM, and improved fat grafting techniques. However, more long-term outcomes of prepectoral breast reconstructions, especially with PMRT, are required, along with studies of the mechanisms allowing for decreases in capsular contracture with ADMs.
Conclusion
-
No potential conflicts of interest relevant to this article was reported.
-
Patient consent
Patient provided written consent for the use of her images.
Notes
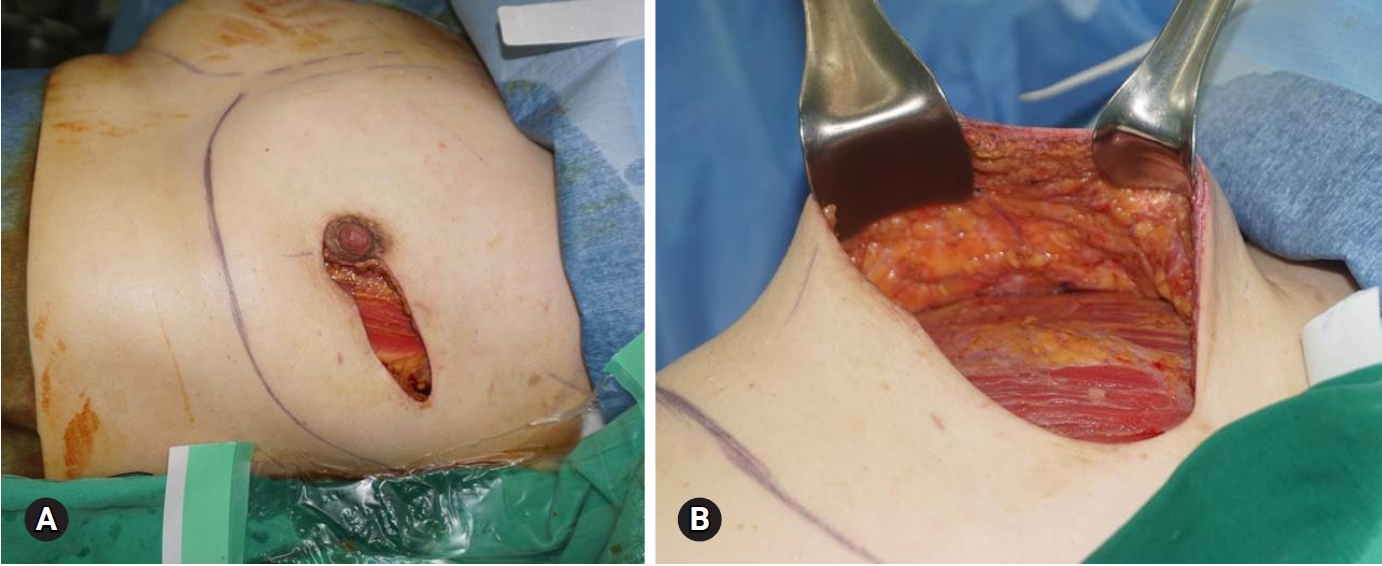
Fig. 1.The mastectomy skin flap has good vascularity (A), and a moderate amount of fat tissue is preserved (B).
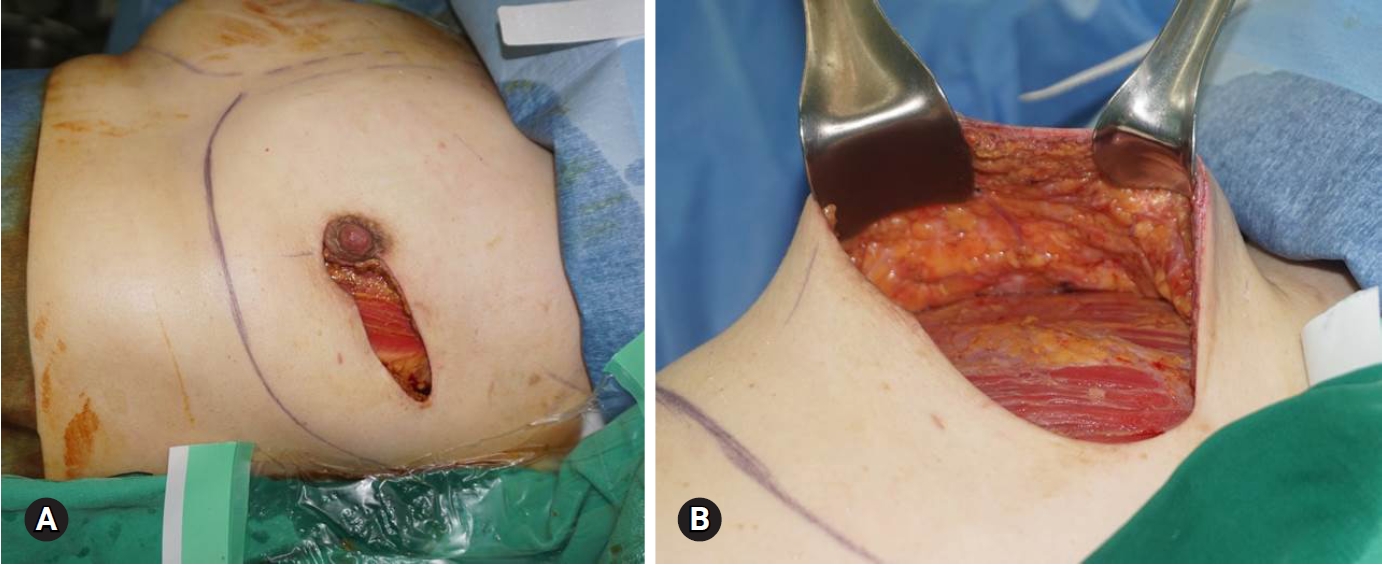

Fig. 2.Two sheets of fenestrated acellular dermal matrices are sewn together and draped over the implant (A). On the back, the implant is partially covered (B).
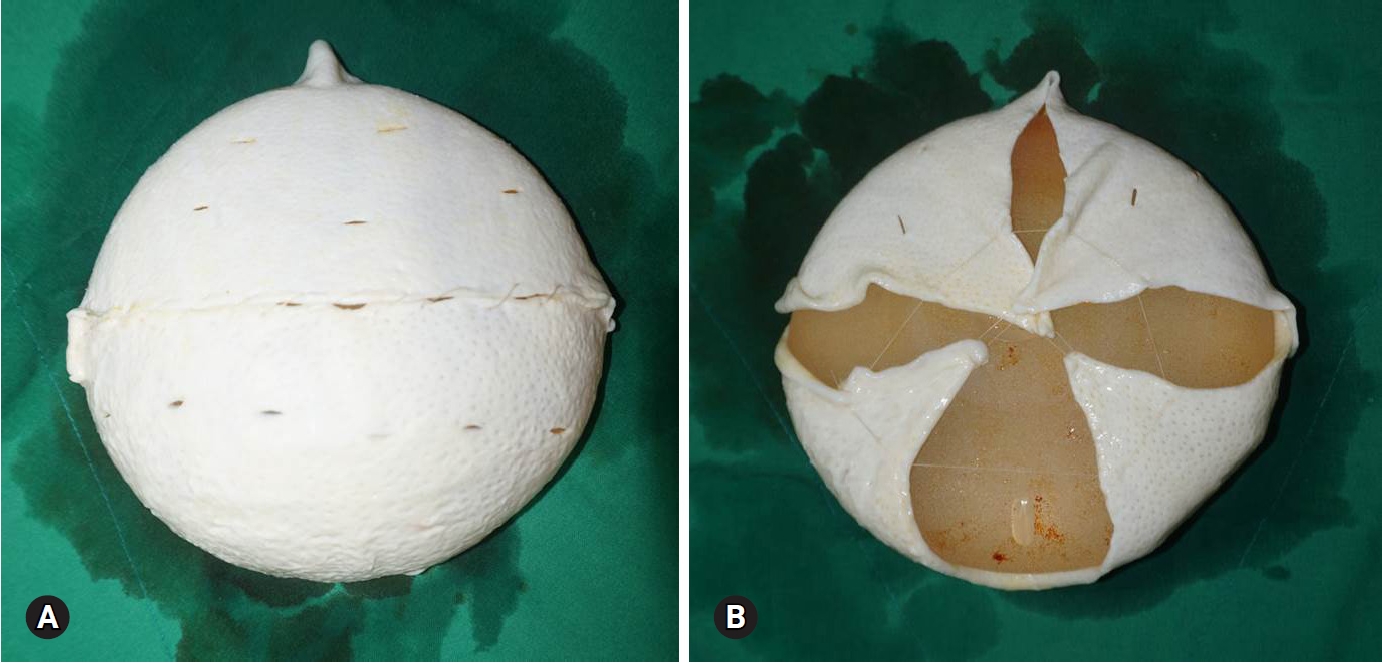

Fig. 3.The device is fully covered by 2 sheets of acellular dermal matrices (A). On the back, the implant is fully covered (B).

Table 1.Indications of prepectoral reconstruction
| Good perfusion of the mastectomy skin flaps |
| Athletes who require extensive pectoralis major use |
| Grade 1 or 2 ptosis, or volume of mastectomy specimen <500 g |
| Low BMI (<35 kg/m2) |
| Non- or ex-smokers |
Table 2.Contraindications of prepectoral reconstruction
- 1. American Society of Plastic Surgeons. 2018 Plastic surgery statistics report [Internet]. Arlington Heights: American Society of Plastic Surgeons; 2019 [cited 2019 Jun 27]. https://www.plasticsurgery.org/documents/News/Statistics/2018/plastic-surgery-statistics-full-report-2018.pdf.
- 2. Schlenker JD, Bueno RA, Ricketson G, Lynch JB. Loss of silicone implants after subcutaneous mastectomy and reconstruction. Plast Reconstr Surg 1978;62:853–61.ArticlePubMed
- 3. Gruber RP, Kahn RA, Lash H, Maser MR, Apfelberg DB, Laub DR. Breast reconstruction following mastectomy: a comparison of submuscular and subcutaneous techniques. Plast Reconstr Surg 1981;67:312–7.ArticlePubMed
- 4. Puckett CL, Croll GH, Reichel CA, Concannon MJ. A critical look at capsule contracture in subglandular versus subpectoral mammary augmentation. Aesthetic Plast Surg 1987;11:23–8.ArticlePubMedPDF
- 5. Biggs TM, Yarish RS. Augmentation mammaplasty: a comparative analysis. Plast Reconstr Surg 1990;85:368–72.ArticlePubMed
- 6. Spear SL, Pelletiere CV. Immediate breast reconstruction in two stages using textured, integrated-valve tissue expanders and breast implants. Plast Reconstr Surg 2004;113:2098–103.ArticlePubMed
- 7. Serra-Renom JM, Fontdevila J, Monner J, Benito J. Mammary reconstruction using tissue expander and partial detachment of the pectoralis major muscle to expand the lower breast quadrants. Ann Plast Surg 2004;53:317–21.ArticlePubMed
- 8. Hammond DC, Capraro PA, Ozolins EB, Arnold JF. Use of a skin-sparing reduction pattern to create a combination skin-muscle flap pocket in immediate breast reconstruction. Plast Reconstr Surg 2002;110:206–11.ArticlePubMed
- 9. Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns 1995;21:243–8.ArticlePubMed
- 10. Salzberg CA. Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann Plast Surg 2006;57:1–5.ArticlePubMed
- 11. Kim IK, Park SO, Chang H, Jin US. Inhibition mechanism of acellular dermal matrix on capsule formation in expander-implant breast reconstruction after postmastectomy radiotherapy. Ann Surg Oncol 2018;25:2279–87.ArticlePubMedPDF
- 12. Vidya R, Iqbal FM. A guide to prepectoral breast reconstruction: a new dimension to implant-based breast reconstruction. Clin Breast Cancer 2017;17:266–71.ArticlePubMed
- 13. Ter Louw RP, Nahabedian MY. Prepectoral breast reconstruction. Plast Reconstr Surg 2017;140(5 Suppl):51S–9S.ArticlePubMed
- 14. Storm-Dickerson T, Sigalove N. Prepectoral breast reconstruction: the breast surgeon's perspective. Plast Reconstr Surg 2017;140(6 Suppl):43S–8S.ArticlePubMed
- 15. Spear SL, Boehmler JH, Bogue DP, Mafi AA. Options in reconstructing the irradiated breast. Plast Reconstr Surg 2008;122:379–88.ArticlePubMed
- 16. Sigalove S, Maxwell GP, Sigalove NM, Storm-Dickerson TL, Pope N, Rice J, et al. Prepectoral implant-based breast reconstruction and postmastectomy radiotherapy: short-term outcomes. Plast Reconstr Surg Glob Open 2017;5:e1631.ArticlePubMedPMC
- 17. Sinnott CJ, Persing SM, Pronovost M, Hodyl C, McConnell D, Ott Young A. Impact of postmastectomy radiation therapy in prepectoral versus subpectoral implant-based breast reconstruction. Ann Surg Oncol 2018;25:2899–908.ArticlePubMedPDF
- 18. Sbitany H, Gomez-Sanchez C, Piper M, Lentz R. Prepectoral breast reconstruction in the setting of postmastectomy radiation therapy: an assessment of clinical outcomes and benefits. Plast Reconstr Surg 2019;143:10–20.ArticlePubMed
- 19. Gabriel A, Maxwell GP. Implant selection in the setting of prepectoral breast reconstruction. Gland Surg 2019;8:36–42.ArticlePubMedPMC
- 20. Salibian AH, Harness JK, Mowlds DS. Staged suprapectoral expander/implant reconstruction without acellular dermal matrix following nipple-sparing mastectomy. Plast Reconstr Surg 2017;139:30–9.ArticlePubMed
- 21. Kobraei EM, Cauley R, Gadd M, Austen WG Jr, Liao EC. Avoiding breast animation deformity with pectoralis-sparing subcutaneous direct-to-implant breast reconstruction. Plast Reconstr Surg Glob Open 2016;4:e708.ArticlePubMedPMC
- 22. Reitsamer R, Peintinger F. Prepectoral implant placement and complete coverage with porcine acellular dermal matrix: a new technique for direct-to-implant breast reconstruction after nipple-sparing mastectomy. J Plast Reconstr Aesthet Surg 2015;68:162–7.ArticlePubMed
- 23. Woo A, Harless C, Jacobson SR. Revisiting an old place: single-surgeon experience on post-mastectomy subcutaneous implant-based breast reconstruction. Breast J 2017;23:545–53.ArticlePubMed
- 24. Eskenazi LB. New options for immediate reconstruction: achieving optimal results with adjustable implants in a single stage. Plast Reconstr Surg 2007;119:28–37.ArticlePubMed
- 25. Glasberg SB. The economics of prepectoral breast reconstruction. Plast Reconstr Surg 2017;140(6 Suppl):49S–52S.ArticlePubMed
- 26. Radovan C. Breast reconstruction after mastectomy using the temporary expander. Plast Reconstr Surg 1982;69:195–208.ArticlePubMed
- 27. Frey JD, Alperovich M, Weichman KE, Wilson SC, Hazen A, Saadeh PB, et al. Breast reconstruction using contour fenestrated alloderm: does improvement in design translate to improved outcomes? Plast Reconstr Surg Glob Open 2015;3:e505.ArticlePubMedPMC
- 28. Sigalove S. Options in acellular dermal matrix-device assembly. Plast Reconstr Surg 2017;140(6 Suppl):39S–42S.ArticlePubMed
- 29. Macadam SA, Lennox PA. Acellular dermal matrices: use in reconstructive and aesthetic breast surgery. Can J Plast Surg 2012;20:75–89.ArticlePubMedPMC
- 30. Sigalove S, Maxwell GP, Sigalove NM, Storm-Dickerson TL, Pope N, Rice J, et al. Prepectoral implant-based breast reconstruction: rationale, indications, and preliminary results. Plast Reconstr Surg 2017;139:287–94.ArticlePubMed
- 31. Zhu L, Mohan AT, Abdelsattar JM, Wang Z, Vijayasekaran A, Hwang SM, et al. Comparison of subcutaneous versus submuscular expander placement in the first stage of immediate breast reconstruction. J Plast Reconstr Aesthet Surg 2016;69:e77–86.ArticlePubMed
- 32. Bernini M, Calabrese C, Cecconi L, Santi C, Gjondedaj U, Roselli J, et al. Subcutaneous direct-to-implant breast reconstruction: surgical, functional, and aesthetic results after long-term follow-up. Plast Reconstr Surg Glob Open 2016;3:e574.ArticlePubMedPMC
- 33. Schaeffer CV, Dassoulas KR, Thuman J, Campbell CA. Early functional outcomes after prepectoral breast reconstruction: a case-matched cohort study. Ann Plast Surg 2019;82(6S Suppl 5):S399–403.ArticlePubMed
- 34. Darrach H, Kraenzlin F, Khavanin N, Chopra K, Sacks JM. The role of fat grafting in prepectoral breast reconstruction. Gland Surg 2019;8:61–6.ArticlePubMedPMC
- 35. Sbitany H, Piper M, Lentz R. Prepectoral breast reconstruction: a safe alternative to submuscular prosthetic reconstruction following nipple-sparing mastectomy. Plast Reconstr Surg 2017;140:432–43.ArticlePubMed
- 36. Cattelani L, Polotto S, Arcuri MF, Pedrazzi G, Linguadoca C, Bonati E. One-step prepectoral breast reconstruction with dermal matrix-covered implant compared to submuscular implantation: functional and cost evaluation. Clin Breast Cancer 2018;18:e703–11.ArticlePubMed
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- The current use of tissue expanders in breast reconstruction: device design, features, and technical considerations
Min-Jeong Cho, Rana V. Farhadi, David W. Nash, Joseph Kaleeny, Stephen P. Povoski, Albert H. Chao
Expert Review of Medical Devices.2024; 21(1-2): 27. CrossRef - The Effect of Early Cultures and Dual-port Expanders on Two-stage, Prepectoral Breast Reconstruction: The 25/25 Study
Hunter R. Moyer, Kayla M. Sisson
Plastic and Reconstructive Surgery - Global Open.2024; 12(1): e5507. CrossRef - Current Global Trends in Prepectoral Breast Reconstruction
Saima Taj, Ravi Chandavarkar, Raghavan Vidya
Medicina.2024; 60(3): 431. CrossRef - Mastectomy with one-stage or two-stage reconstruction in breast cancer: analysis of early outcomes and patient’s satisfaction
Angela Gurrado, Alessandro Pasculli, Alessia Toma, Michele Maruccia, Rossella Elia, Marco Moschetta, Michele Telegrafo, Giuseppe Massimiliano De Luca, Walter Lavermicocca, Elisabetta Poli, Francesco Paolo Prete, Lucia Ilaria Sgaramella, Giuseppe Giudice,
Updates in Surgery.2023; 75(1): 235. CrossRef - Immediate prepectoral breast reconstruction using an ADM with smooth round implants: A prospective observational cohort study
Fabio Santanelli di Pompeo, Guido Firmani, Guido Paolini, Vittoria Amorosi, Francesca Briganti, Michail Sorotos
Journal of Plastic, Reconstructive & Aesthetic Surgery.2023; 80: 56. CrossRef - Optimizing Prepectoral Implant Placement and Concomitant Fat Grafting After Tissue Expansion
Alisa O. Girard, Christopher D. Lopez, Christina M. Ambrosino, Kristen P. Broderick
Annals of Plastic Surgery.2023; 90(6S): S483. CrossRef - A Propensity Score–Matched Comparison of Perioperative Outcomes in Prepectoral Smooth Versus Textured Tissue Expander Breast Reconstruction
Kevin Perez, Pope Rodnoi, Sumeet S. Teotia, Nicholas T. Haddock
Annals of Plastic Surgery.2023; 90(5S): S242. CrossRef - Prepectoral Breast Reconstruction and Quality of Life: One Step Further
Michael Kontos
Journal of Investigative Surgery.2022; 35(4): 848. CrossRef - Selective Denervation of Pectoralis Major Muscle Improves Cosmetic Outcome and Quality of Life in Retro-Pectoral Implant Based Breast Reconstruction
Marco Bernini, Silvia Sordi, Niccolo’ Bembi, Icro Meattini, Diego De Benedetto, Jacopo Nori Cucchiari, Lorenzo Livi, Lorenzo Orzalesi
Clinical Breast Cancer.2022; 22(1): 60. CrossRef - Meshed Acellular Dermal Matrix for Two-Staged Prepectoral Breast Reconstruction: An Institutional Experience
Jessica Luo, Rhett N. Willis, Suzanna M. Ohlsen, Meghan Piccinin, Neal Moores, Alvin C. Kwok, Jayant P. Agarwal
Archives of Plastic Surgery.2022; 49(02): 166. CrossRef - Chances and challenges—analysis of trends in breast reconstruction
Siling Yang, Xixi Lin, Maximilian Kückelhaus, Tobias Hirsch, Marie-Luise Klietz, Matthias M. Aitzetmüller
Journal of Plastic, Reconstructive & Aesthetic Surgery.2022; 75(8): 2584. CrossRef - Cost analysis of pre-pectoral implant-based breast reconstruction
Sachin Chinta, Daniel J. Koh, Nikhil Sobti, Kathryn Packowski, Nikki Rosado, William Austen, Rachel B. Jimenez, Michelle Specht, Eric C. Liao
Scientific Reports.2022;[Epub] CrossRef - Awake breast cancer surgery: strategy in the beginning of COVID-19 emergency
Gianluca Vanni, Marco Pellicciaro, Marco Materazzo, Mario Dauri, Rolando Maria D’angelillo, Chiara Buonomo, Adriano De Majo, Chiara Pistolese, Ilaria Portarena, Alessandro Mauriello, Francesca Servadei, Erica Giacobbi, Agostino Chiaravalloti, Oreste Claud
Breast Cancer.2021; 28(1): 137. CrossRef - Next-generation surgical meshes for drug delivery and tissue engineering applications: materials, design and emerging manufacturing technologies
Francesca Corduas, Dimitrios A. Lamprou, Elena Mancuso
Bio-Design and Manufacturing.2021; 4(2): 278. CrossRef - Examining the Effects of Postmastectomy Radiation Therapy in Prepectoral Versus Subpectoral Autologous Breast Reconstruction
Ashraf A. Patel, Connor P. Arquette, Pooja S. Yesantharao, Mimi R. Borrelli, Kristen P. Broderick, Jennifer E. Cheesborough, Gordon K. Lee, Rahim S. Nazerali
Annals of Plastic Surgery.2021; 86(5S): S390. CrossRef - Day-case approach to immediate breast reconstruction: pushing the boundaries of ambulatory breast surgery in the post-COVID-19 era
H Shaker, NAR Leena, V Mayers, F Koussa, A Deshpande
The Annals of The Royal College of Surgeons of England.2021; 103(6): 426. CrossRef - Meta-analysis of prepectoral implant-based breast reconstruction: guide to patient selection and current outcomes
Olivia Abbate, Nikki Rosado, Nikhil Sobti, Brittany L. Vieira, Eric C. Liao
Breast Cancer Research and Treatment.2020; 182(3): 543. CrossRef

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite