Differential diagnosis of motor weakness in the right lower limb of a 59-year-old male patient
Article information
Patient information
A 59-year-old man visited the spine center of Yeungnam University Hospital for motor weakness in the right ankle, which started approximately 1 year earlier and slowly worsened. At first, the ankle and toe dorsiflexion weakened. The weakness then gradually spread to more proximal muscles of the right lower limb. The patient did not experience any sensory changes or pain. There was no history of trauma related to motor weakness; however, he had been told that he had a spinal deformity in the lumbar vertebrae since childhood. He had no medical history and was not taking any medications.
Clinical findings
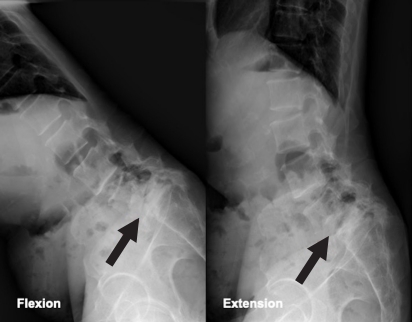
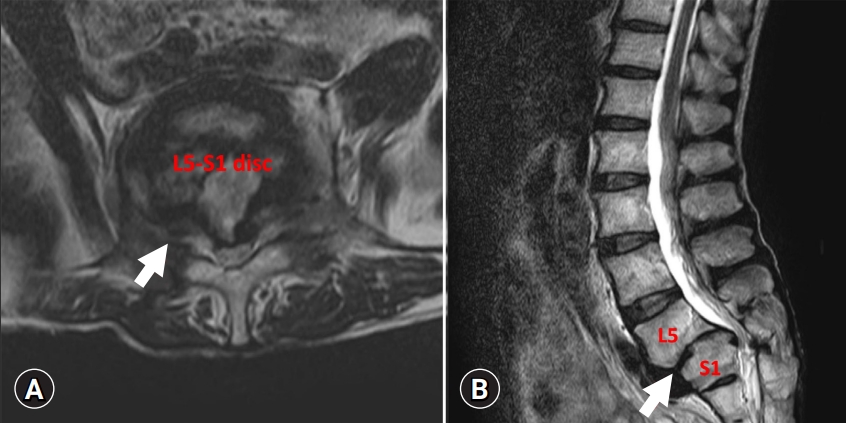
On physical examination, muscle atrophy of the right lower extremity and fasciculations at multiple sites were observed. However, tongue fasciculations were not observed. In the manual muscle-strength test, the right knee flexors and extensors were found to be 3/5, and the right ankle dorsiflexors and plantar flexors were found to be 1/5 and 2/5, respectively, on the Medical Research Council scale for muscle strength. Muscle strength of both the upper extremities and left lower extremity was normal. Light touch and pin-prick sensations were normal. When deep tendon reflexes were examined, the left knee-jerk reflex was normal but the right knee-jerk reflex was decreased. Hoffmann sign and ankle clonus were not noted. Before visiting our hospital, the patient had visited a local hospital where he was diagnosed with spondylolisthesis at L5–S1 based on a plain radiograph of the lumbar spine (Fig. 1). The lumbar spine magnetic resonance imaging (MRI) performed in the local hospital revealed spondylolisthesis at L5–S1, mild central spinal stenosis at L5–S1, foraminal stenosis at right L5–S1, and disc degeneration at L4–S1 (Fig. 2).
Differential diagnosis
The following diagnoses were considered.
1. Right L5 and S1 radiculopathy
The patient was diagnosed with spondylolisthesis at L5–S1 and foraminal stenosis at right L5–S1, which may have resulted in right L5 and S1 radiculopathy. In addition, the right ankle weakness in the patient was consistent with the L5 and S1 myotome. However, more extensive clinical involvement of the myotome than can be explained by the findings of the patient’s lumbar MRI, as well as the absence of sensory symptoms and radiating pain, make this diagnosis less likely.
2. Amyotrophic lateral sclerosis (ALS)
The clinical spectrum of ALS comprises varying degrees of lower motor neuron (LMN) and upper motor neuron involvement [1]. Progressive, often asymmetric weakness in the absence of prominent sensory changes is a characteristic symptom of ALS. The insidious and progressive motor weakness, involving more than one myotome or peripheral nerve territory, in the right lower limb of the patient is suggestive of ALS. In addition, fasciculation of the leg muscles supported this diagnosis. Since only LMN signs were found upon physical examination, a detailed, comprehensive electrophysiologic study and clinical follow-up were necessary to confirm the diagnosis.
3. Multifocal motor neuropathy (MMN)
MMN is a rare autoimmune motor neuropathy that causes slowly progressive asymmetric distal weakness, especially in the upper extremities [2]. Although lower limb involvement has been reported in approximately 10% of cases, it is much rarer than upper limb involvement, as predominant upper limb involvement is a supportive clinical criterion for diagnosing MMN [2]. The fact that marked muscle wasting is rare in the early symptomatic phase could be a useful point for differentiation. The presence of a conduction block in electrophysiologic studies may be another finding for diagnosis.
4. Post-polio syndrome
This syndrome results from the chronic neuromuscular sequelae of acute poliomyelitis [3]. Approximately 25% to 60% of patients with a history of acute poliomyelitis have been reported to develop subsequent neuromuscular symptoms decades after acute infection. The main symptom of this syndrome is new, persistent, and progressive muscle weakness. Myalgia, cramps, and fasciculations are also commonly observed. However, since the patient did not have a history of poliomyelitis, it was unlikely to be the cause of his symptoms.
5. Chronic inflammatory demyelinating polyneuropathy (CIDP)
This is an acquired, immune-mediated peripheral neuropathy characterized by demyelination. Among several subtypes of CIDP, a motor-predominant form without significant sensory symptoms could have caused symptoms similar to those of the patient, although this form is reportedly rare (<10% of CIDP cases) [4]. However, considering the asymmetric involvement of the patient’s lower limbs, the possibility of CIDP was low. Nerve conduction studies and cerebrospinal fluid analysis are required for positive diagnosis.
6. Spinobulbar muscular atrophy
This is an X-linked disorder caused by cytosine-adenine-guanine trinucleotide repeat expansion in the androgen receptor gene on the X chromosome, thus affecting only males [5]. Slowly progressive motor weakness, often accompanied by prominent cramps, usually begins in the bulbar muscles or lower limb muscles. Gynecomastia and testicular atrophy can also occur. Since the patient did not have endocrine abnormalities or bulbar and perioral muscle involvement, the possibility of this disorder was low.
7. Intracranial lesions
Stroke or brain tumors should be considered in the differential diagnosis of limb weakness. In this patient, stroke could be excluded considering the presentation of insidious progressive weakness. In addition, considering the LMN signs, it is unlikely that our patient had this disorder.
8. Inclusion body myositis
This is a disease that shows gradual asymmetrical muscle wasting in patients over 50 years old [6]. However, normal or exaggerated reflexes are observed with this disease, and visible fasciculation is not seen although fasciculations can be detected by electromyography (EMG) in up to 40% of patients [6]. None of these characteristics were observed in the patient.
9. Thyrotoxicosis
Thyrotoxicosis may be accompanied by fasciculations and muscle weakness. However, the possibility of this diagnosis was considered low because the patient did not have systemic symptoms of thyrotoxicosis, such as heat intolerance and tachycardia.
10. Paraneoplastic syndrome
Subacute sensory neuropathy is a typical feature of paraneoplastic syndrome; however, symptoms may appear as sensorimotor neuropathies such as brachial plexopathy or Guillain-Barré syndrome [7]. To exclude the possibility of this disease, abdominal and pelvic computed tomography (CT) scans are required.
Diagnostic assessment
In the electrophysiological study, the amplitude of compound muscle action potentials of the right peroneal nerve was decreased, and there were no abnormal findings in the sensory nerve action potential (Table 1). In needle EMG, positive sharp waves and fibrillation potentials of varying degrees were found in the cervical, thoracic, and lumbar paraspinal muscles and muscles of all four extremities, and long-duration complex motor-unit action potentials with reduced interference patterns were found in the muscles of the right lower limb. The bulbar muscles were preserved, and myopathic discharges or early recruitment patterns were not observed in any of the examined muscles. In addition, the serum creatine phosphokinase level slightly increased to 235 IU/L. Except for this, no abnormal findings were found in the laboratory tests, including complete blood count, C-reactive protein level, erythrocyte sedimentation rate, thyroid function test, and liver and kidney functions. No abnormal findings were observed on brain CT scan images.
Based on these findings, the patient was diagnosed with LMN-predominant ALS according to the revised El Escorial criteria-2015 [8], and all other disorders were ruled out.
Discussion
ALS is diagnosed when “progressive upper and LMN deficits in at least one limb or region of the human body” and/or “LMN deficits as defined by clinical examination (one region) and/or by EMG in two body regions (defined as bulbar, cervical, thoracic, lumbosacral)” are present [8]. However, early-stage ALS is difficult to differentiate from other diseases that may show similar clinical symptoms, especially radiculopathy [9]. Considering that misdiagnosis of cervical or lumbar radiculopathy might result in unnecessary surgeries, which are known to significantly worsen the prognosis of ALS, a correct diagnosis of ALS is of great importance. In an effort to differentiate early stages of ALS from other disorders with similar presentations, the concept of split-leg sign (prominent wasting of ankle plantar flexors compared to ankle dorsiflexors) was proposed [10]. However, this concept is still controversial [6]; our patient showed greater motor weakness in his ankle dorsiflexors than in his ankle plantar flexors.
Currently, there is no cure for ALS. However, early diagnosis is important to reduce uncertainty and thus the patient’s distress and anxiety, even though the disease prognosis is poor. This prevents unnecessary examinations and surgeries, facilitates making future medical plans, and provides opportunities for enrollment in clinical trials and for treatment with neuroprotective agents earlier when fewer motor neurons have degenerated. In addition, it is known that timely initiation of respiratory support using non-invasive or invasive ventilation and securing nutrient supply through feeding gastrostomy improves the survival and quality of life of patients with ALS.
Conclusion
When clinicians encounter patients with progressive motor weakness without sensory symptoms, they should always keep in mind the possibility of ALS. Accurate diagnosis of ALS prevents patients from receiving unnecessary treatment and greatly contributes to improvements in life expectancy and quality of life.
Notes
Ethical statements
This study was conducted in accordance with the Declaration of Helsinki and was reviewed and approved by the Institutional Review Board (IRB) of Yeungnam University Hospital (IRB No: 2022-08-030). Written consent was obtained from the patient.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: Investigation, Formal analysis, Methodology, Project administration, Visualization: all authors; Data curation: JHB; Supervision: SK; Writing-original draft: all authors; Writing-review & editing: all authors.