Intraabdominal abscess mimicking gastric cancer recurrence: a case report
Article information
Abstract
Surgical site infection is a common healthcare-associated infection that rarely occurs several months after surgery. Herein, a case is described in which an abdominal mass lesion was found at a 6-month follow-up visit after gastrectomy was performed for early gastric cancer. Positron emission tomography-computed tomography revealed a 2.5 cm-sized mass with a high maximal standard uptake value (8.32), located above a previous anastomosis site. Locoregional recurrence of gastric cancer was diagnosed by multidisciplinary team discussion, and explorative laparotomy was performed. However, surgical and pathologic findings revealed that the mass was an intraabdominal abscess. In conclusion, differential diagnosis of delayed abscess formation should be considered if the possibility of tumor recurrence is low, especially after early gastric cancer surgery.
Introduction
Surgical site infection (SSI) is a common healthcare-associated infection that prolongs hospital stay and increases postoperative morbidity and mortality. Therefore, it is important to know the risk factors for SSI and manage preventable factors. If an SSI occurs, early diagnosis and treatment are important. SSI occurs mostly within 30 days after surgery, but it can also rarely occur several months after surgery [1].
Intraabdominal abscess (IAA) is an organ or space type SSI. Patients with postoperative IAA usually show symptoms such as abdominal pain and fever, along with physical examination findings such as direct or rebound tenderness and laboratory findings such as leukocytosis and increased C-reactive protein (CRP). IAA can be easily diagnosed using computed tomography (CT), ultrasonography, and magnetic resonance imaging. However, in some cases, an abscess may be mistaken for a tumor. Therefore, differential diagnosis is necessary for correct treatment and subsequent good prognosis of patients [2-4].
We present a case in which a mass lesion located above the previous anastomosis site was found at a 6-month follow-up study after gastrectomy for early gastric cancer (T1bN0M0, stage Ia). After further evaluation and multidisciplinary team discussion, locoregional recurrence of gastric cancer was diagnosed, and surgery was performed. However, surgical findings and pathologic examination revealed that the mass was an IAA misdiagnosed as a tumor.
Case
Ethical statements: This study was approved by the Institutional Review Board (IRB) of Yeungnam University Hospital (IRB No: YUMC-2022-10-041), and informed consent was obtained from the patient.
A 65-year-old male patient underwent abdominal CT 6 months after gastrectomy that revealed a mass lesion suggestive of local recurrence (Fig. 1). He had a medical history of hypertension, diabetes mellitus, and surgical history of totally laparoscopic distal gastrectomy for early gastric cancer (T1bN0M0, stage Ia) 6 months prior and transurethral resection of bladder tumor for bladder cancer 9 years prior. His vital signs were stable without fever, and physical examination revealed no tenderness or rebound tenderness other than the previous surgical scar. Laboratory examination showed no leukocytosis or CRP increase, and the levels of tumor markers such as carbohydrate antigen 19-9 and carcinoembryonic antigen were within the normal range.
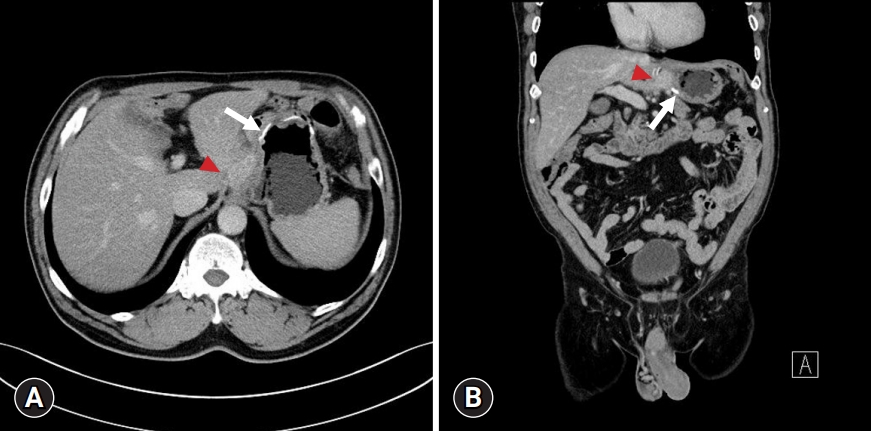
Computed tomography finding of the patient. Both (A) axial and (B) coronal views show a conglomerated mass (arrowheads) located above the anastomosis site (white stapling lines, arrows) and located between the liver and the remnant stomach.
Abdominal CT revealed a conglomerated mass lesion between the remnant lesser curvature of the stomach above the previous anastomosis site and the left lobe of the liver. Esophagogastroduodenoscopy showed no abnormalities, and positron emission tomography (PET)-CT showed a 2.5 cm-sized 18F-fluorodeoxyglucose (FDG)-avid lesion (maximal standard uptake volume [SUVmax], 8.32) without metastasis to other organ or peritoneal seeding (Fig. 2). Based on the above findings, a multidisciplinary team discussion was held, and surgery was performed for definite diagnosis and treatment of local recurrence of early gastric cancer.

Positron emission tomography-computed tomography finding of the patient. A 2.5 cm-sized 18F-fluorodeoxyglucose-avid lesion (arrow; maximal standard uptake volume, 8.32) without other organ metastasis or peritoneal seeding is observed.
Explorative laparotomy was commenced, and adhesiolysis was performed to access the mass lesion. During peripheral dissection, pus was drained from the mass and cultured to identify the organism. After dissecting the mass from the remnant stomach wall and surrounding tissues, frozen biopsy was performed. No malignant cells were observed except for fibrosis with lymphocyte aggravation. Hence, the operation was terminated without resection of remnant stomach.
The results of the drained pus culture showed no growth of organisms, and the pathologic result of the mass lesion showed acute and chronic inflammation with abscess formation and fibrosis. The patient was discharged on day 10 after surgery without any complications except for wound reclosure due to seroma (Fig. 3).
Discussion
Risk factors for SSI can be classified into patient-related, surgical, and physiological or environmental factors. Patient-related factors include existing infection, advanced age, obesity, smoking, diabetes mellitus, medication (e.g., chemotherapy or steroid), American Society of Anesthesiologists physical status classification, preoperative hemoglobin/albumin level, and radiation exposure. Surgical factors include the duration of surgery, operative procedure, inadequate surgical scrubbing, use of prophylactic antibiotics, wound contamination class, urgency of surgery (emergency or elective), surgical approach (open or laparoscopic), drainage insertion, and presence of implant. Lastly, physiological or environmental factors include trauma, shock, blood transfusion, hypothermia, hypoxia, and hyperglycemia [5,6].
In this case, the relevant risk factors for SSI were advanced age (65 years), obesity (body mass index of 28.3 kg/m2), and diabetes mellitus as patient-related factors and duration of surgery (240 minutes), operative procedure (gastrectomy), and drainage tube insertion as surgical risk factors, with no environmental factors. Although it can be included in several categories concerning risk factors for SSI, gastrectomy with drainage tube insertion is a common procedure in gastric cancer patients with diabetes mellitus. Also, in this case, the patient showed no signs of SSI until 30 days after surgery. In addition, at the follow-up visit 6 months after gastrectomy, vital signs were stable without fever, physical examination findings were nonspecific, and there was no increase in white blood cells or CRP on the laboratory findings. Abdominal CT revealed a mass with contrast enhancement above the previous anastomosis site, a common site of locoregional recurrence of gastric cancer. In this context, it was difficult to suspect IAA, given that the mass lesion on CT did not show any findings of abscess such as air or fluid collection within the mass, heterogenous enhancement, adjacent inflammatory stranding, and that the lesion occurred 6 months after surgery [7,8].
The recurrence pattern of gastric cancer after surgery is classified into locoregional, peritoneal, and distant or hematogenous recurrence, which may show a mixed pattern. Early gastric cancer has a low recurrence rate of 1.5% but follows the same recurrence pattern with various intervals ranging from 1.5 to 69.5 months, and the risk factors for recurrence include submucosal invasion and lymph node metastasis [9,10]. In this case, submucosal invasion (T1b) was present, but lymph node metastasis and lymphatic/vascular/neural invasion were negative. Therefore, there was no risk factor for recurrence except for submucosal invasion, although the SUVmax of the mass lesion on PET-CT was 8.32, suggesting locoregional recurrence due to micrometastasis.
Usually, organisms inside the abscess cavity are polymicrobial and it is difficult to culture and identify them using conventional culture-based methods. Therefore, it is common to obtain a negative culture result from the abscess cavity [11,12]. In this case, the drained pus showed negative result.
The pathological result of the resected mass lesion revealed acute and chronic inflammation with abscess formation and fibrosis. As previously mentioned, most SSIs occur within 30 days of surgery; however, in rare cases, SSIs can occur even several months after surgery [1]. In some cases, an abscess can be misdiagnosed as a tumor, as in this case [2-4]. Therefore, differential diagnosis for an abscess should be considered when the probability of tumor recurrence is low.
In conclusion, delayed abscess formation after gastric cancer surgery can be mistaken for tumor recurrence. Differential diagnosis for an abscess is required, especially if recurrence is suspected after early gastric cancer without lymph node metastasis.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.