PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(Suppl); 2023 > Article
-
Original article
Cytotoxicity of dental self-curing resin for a temporary crown: an in vitro study -
Jae-wan Ko1,2
 , Joon Sakong3,4
, Joon Sakong3,4 , Sohee Kang5
, Sohee Kang5
-
Journal of Yeungnam Medical Science 2023;40(Suppl):S1-S8.
DOI: https://doi.org/10.12701/jyms.2023.00080
Published online: April 26, 2023
1Department of Dental Technology, Daegu Health College, Daegu, Korea
2Department of Public Health, Graduate School of Environment and Public Health Studies, Yeungnam University, Daegu, Korea
3Department of Occupational and Environmental Medicine, Yeungnam University Hospital, Daegu, Korea
4Department of Preventive Medicine and Public Health, Yeungnam University College of Medicine, Daegu, Korea
5Department of Dentistry, Yeungnam University College of Medicine, Daegu, Korea
- Corresponding author: Sohee Kang, DDS, PhD Department of Dentistry, Yeungnam University College of Medicine, 170 Hyeonchung-ro, Nam-gu, Daegu 42415, Korea Tel: +82-53-620-3282 • Fax: +82-53-629-1772 • E-mail: kangsh@yu.ac.kr
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,509 Views
- 76 Download
Abstract
-
Background
- Residual monomer tests using high-performance liquid chromatography and cytotoxicity tests were performed to analyze the effect on the oral mucosa of a self-curing resin for provisional crown production.
-
Methods
- A cytotoxicity test was performed to confirm whether leaked residual monomers directly affected oral mucosal cells. The cytotoxicity of the liquid and solid resin polymers was measured using a water-soluble tetrazolium (WST) test and microplate reader.
-
Results
- In the WST assay using a microplate reader, 73.4% of the cells survived at a concentration of 0.2% liquid resin polymer. The cytotoxicity of the liquid resin polymer was low at ≤0.2%. For the solid resins, when 100% of the eluate was used from each specimen, the average cell viability was 91.3% for the solid resin polymer and 100% for the hand-mixed self-curing resin, which is higher than the cell viability standard of 70%. The cytotoxicity of the solid resin polymer was low.
-
Conclusion
- Because the polymerization process of the self-curing resin may have harmful effects on the oral mucosa during the second and third stages, the solid resin should be manufactured indirectly using a dental model.
- In dentistry, temporary crowns are manufactured to increase satisfaction with oral aesthetics and functional satisfaction with pronunciation and mastication from the start to the end of treatment [1-4]. The most commonly used material is a self-curing resin, which exhibits good consistency in tooth color and opaqueness, is easily ground and shaped, has high strength, and is inexpensive [5-7].
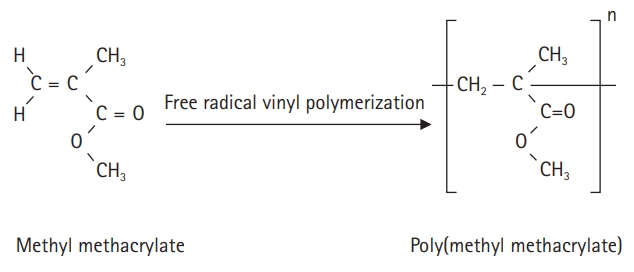
- The self-curing resin for temporary crown production is an acrylic polymer; the polymerization initiator and additives for polymerization are placed in a standard solution of methyl methacrylate (MMA), and benzoyl peroxide is decomposed by the activator. Polymerization is achieved by a reaction with free radicals [8]. When the dedicated powder and MMA solution are mixed, they combine and cure through a self-polymerization process to form poly(methyl methacrylate) (PMMA) (Fig. 1). Monomers that are not polymerized by the self-polymerization method are called residual monomers, which lead to corrosion and physical erosion by saliva over time. This degrades the mechanical properties of the denture base [9].
- The margin of the provisional crown is likely to contact the gingival sulcus epithelium or connective epithelial cells, depending on the type of restoration. Abdallah et al. [10] found that for gingival epithelial cells, PMMA promoted higher cell viability on day 1 than all other biomaterials studied. It was confirmed that amination improved the chemical properties of the PMMA surface, making it more beneficial to teeth and promoting interactions with gingival epithelial cells and fibroblasts. However, in cytotoxicity tests of cell lines with leached MMA in an incompletely polymerized state, Souto-Lopes et al. [11] found that the viability of the groups treated with different concentrations of the chemical agents was significantly lower (p<0.05) than that of the control group.
- Computer-aided design (CAD)/computer-aided manufacturing (CAM) systems have been introduced to design and manufacture restorations in the dental field, and their use for prosthetic manufacturing has increased. In addition, owing to the research and development of three-dimensional–printed dental resins, their use is expanding in the medical and dental fields.
- The self-curing resin used in the CAD/CAM system is formed by mixing and compressing with a machine rather than mixing and polymerizing by hand, the latter of which is a general method whose methodological differentiation increases the density and strength of the finished product when manufacturing temporary crowns [12]. However, few studies have been conducted on the residual monomers of the self-curing resins used in CAD/CAM and their cytotoxicity [13].
- The purpose of this study was to compare residual monomer concentrations and cytotoxicity according to the type of self-curing resin used. First, in the fourth stage of polymerization (rubber stage), the residue of the self-polymerizing resin for CAD/CAM, which is mixed and polymerized by machine, was completely polymerized according to International Organization for Standardization (ISO) and compared with self-curing resin where mixed polymerization was performed manually. Second, the amount of residual monomer leaked from the self-curing resin during the stringy stage before complete polymerization for temporary crown fabrication was studied. The cytotoxicity of the liquid and solid resin polymers of the fourth stage installed in the oral cavity after complete polymerization was then evaluated. The purpose of this study is to provide information necessary for improving oral health by reducing the amount of residual monomers during the manufacture of self-curing resins and improving the manufacturing method of these resins.
Introduction
- 1. Residual monomer dilution test
- The materials used in the experiment were a hand-mixed self-curing resin (Vertex Self Curing Powder+Liquid; Vertex-Dental B.V., Soesterberg, The Netherlands) and a CAD/CAM PMMA block (Temp Basic A2-B2 95H16; Zirkonzahn, Gais, Italy). For the experiment, five disc-shaped specimens (50 mm in diameter, 0.5 mm in height) were generated at 25°C for 24 hours.
- Each disk specimen was placed in a 10-mL volumetric flask, and an acetone was added to the flask to a volume of 10 mL. The test solution was sealed and magnetically stirred at 25°C for 72 hours. Two milliliters of the dissolved polymer were added to 8 mL of methanol for precipitation. Five milliliters of the slurry was transferred to a stoppered glass tube and centrifuged for 15 minutes. A 3-mL aliquot of the supernatant was used to measure the residual monomer content by ultra-performance liquid chromatography (UPLC). The test conditions were as follows: ultraviolet absorbance spectrophotometer (measured at 210 nm), ACQUITY UPLC BEH C18 of 1.7 µm (Waters Corp., Milford, MA, USA), 2.1×50-mm column, maintained at 40°C, flow rate of 0.1 min/mL for 30 minutes, and 100% methanol mobile phase.
- 2. Cytotoxicity assessment at each stage
- To examine the cytotoxicity of the specimens in each group, L929 fibroblasts (Mus musculus; mouse subcutaneous connective tissue; areolar and adipose fibroblasts, biosafety level 1; male sex) were used as the cell line according to the ISO (10993-5:2009) standard.
- To confirm the cytotoxicity of the hand-mixed self-curing resin at various stages, the liquid resin polymers for the second stage (stringy stage) and third stage (dough stage) and solid resin polymer for the fourth stage (rubber stage) were used separately for cytotoxicity assessment. The CAD/CAM self-curing resin used was a solid resin.
- L929 cells were inoculated at 1×104 cells per well in a 96-well plate in culture medium and cultured for 24 hours in a CO2 incubator at 37°C, 90% humidity, and 5% CO2 (INCO 246; Memmert, Schwabach, Germany). After mixing the liquid resin polymer with minimum essential medium (MEM) 05 according to ISO (10993-12:2012) standards, sample solutions were prepared at concentrations of 100%, 75%, 65%, 35%, 25%, 10%, 1%, 0.8%, 0.6%, 0.4%, 0.2%, and 0.1%. One hundred microliters of the prepared sample solutions or blank test solutions was added to each well of the cultured cells after removing the existing culture medium.
- After incubation for 4 hours in a CO2 incubator, the sample solution was removed, and each well was washed twice with 100 µL of phosphate-buffered saline. Each well was treated with water-soluble tetrazolium (WST) medium (10% WST in MEM) using the WST method and incubated for 2 hours in a CO2 incubator. The absorbance was measured using a microplate reader (M200 PRO; Tecan, Männedorf, Switzerland) at 450 nm and 625 nm. Six wells were used for each concentration.
- L929 cells were inoculated in a 96-well plate in culture medium at 1×104 cells per well and cultured at 37°C, 90% humidity, and 5% CO2 for 24 hours. The positive control (0.1% zinc diethyldithiocarbamate polyurethane film) and negative control (high-density polyethylene film) were prepared in MEM containing 10% fetal bovine serum and 1% penicillin/streptomycin according to ISO (10993-12:2012) standards. Test sample eluates were prepared at an elution ratio of 0.1 g/mL for the positive/negative controls and 0.2 g/mL for the test samples. After removing the existing culture medium on the L929 cells, the test solution was added with WST medium (10% WST in MEM) and incubated for 2 hours in a CO2 incubator. The absorbance was measured at 450 and 625 nm.
Methods
1) Preparation of dental self-curing resin specimens
2) Residual monomer dilution test
1) Liquid resin experiment
2) Solid resin experiment
- 1. Residual monomer dilution test
- The content of residual monomer was lower for the CAD/CAM self-curing resin (1.07 wt%) than for the hand-mixed self-curing resin (4.48 wt%) (Table 1).
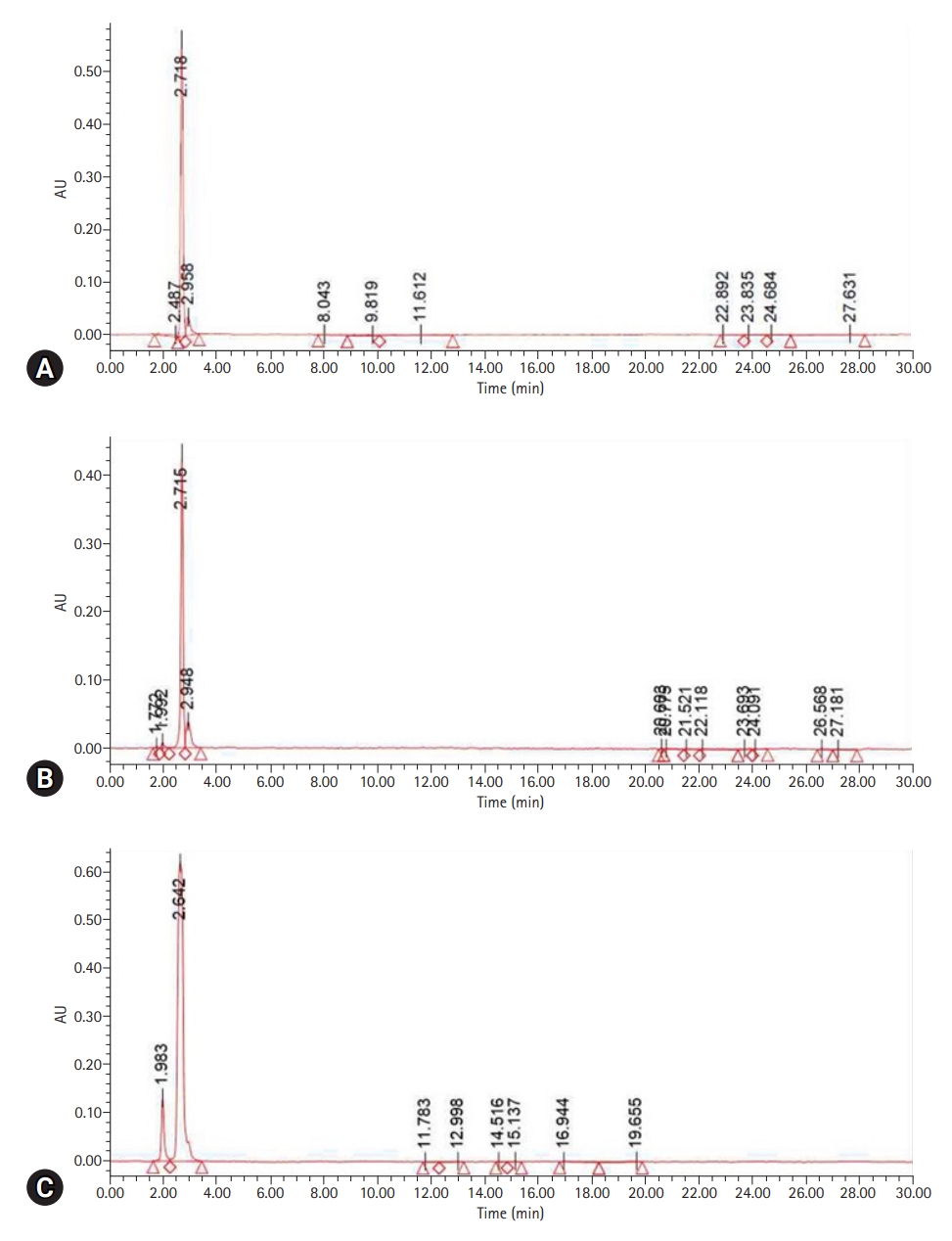
- MMA was detected with a UPLC retention time (RT) of 2.706 minutes. The peak RT of the liquid resin polymer was 2.718 minutes; its peak area was the highest at 3,402,588 mV∙sec and was shown to be the same as the standard. The peak area of the self-curing resin for CAD/CAM was 2,605,091 mV∙sec at an RT of 2.715 minutes, and the peak area of the hand-mixed self-curing resin was 8,883,090 mV∙sec at an RT of 2.642 minutes (Table 1, Fig. 2).
- 2. Cytotoxicity test
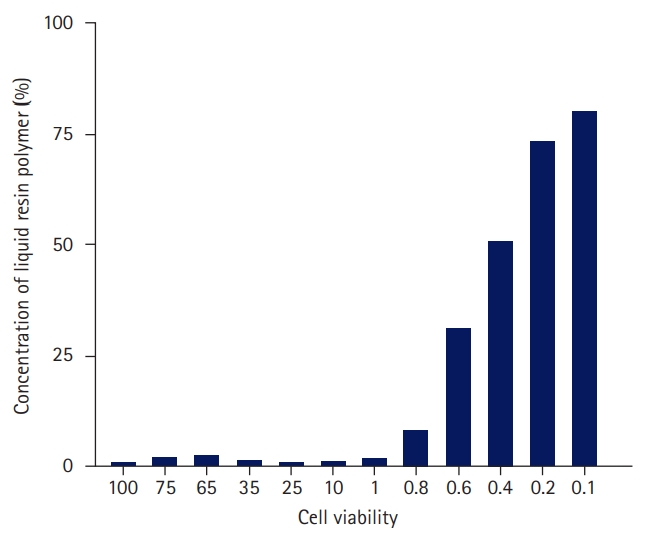
- As a result of measuring the cell viability according to the concentration of the liquid resin polymer six times, the cell viability at each concentration is shown in Table 2 and Fig. 3.
- At a concentration of 0.4% liquid resin polymer, 50.9% of the cells survived; at a concentration of 0.2%, 73.4% of the cells survived; and at a concentration of 0.1%, 80.1% of the cells survived. In the 0.49% concentration standard of liquid resin polymer, cell activity increased as the concentration decreased. In the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide (MTT) assay, the liquid resin polymer was deemed to be cytotoxic at 70% cell viability, which occurred when the concentration of the liquid resin polymer exceeded 0.2% of the elution standard (Table 2, Figs. 3, 4).
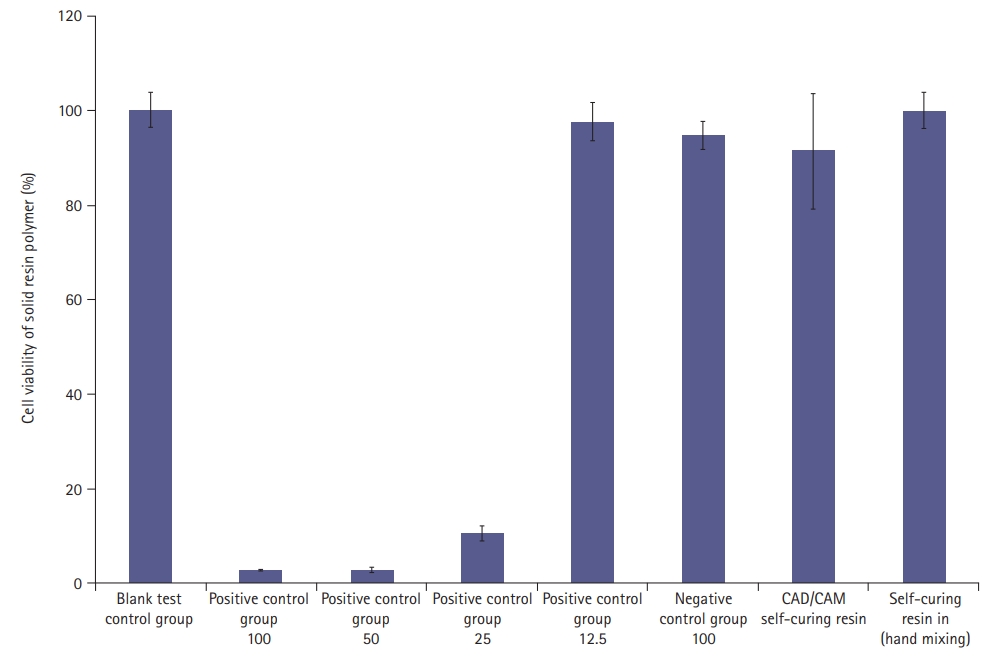
- Using the MTT assay and 100% of the eluate from each specimen, the mean cell viabilities for the CAD/CAM self-curing resin, self-curing resin, and cytotoxicity standard were 91.3%, 100.0%, and 70%, respectively. Therefore, the cytotoxicity of the solid resin polymer was low (Fig. 5).
Results
1) Liquid self-curing resin
2) Solid self-curing resin
- The PMMA resins, polymerized by various methods using chemical catalysts or light and heat in dental medical institutions and dental laboratories, are mainly divided into the photopolymerization, thermal polymerization, and self-polymerization series.
- According to a previous study using liquid chromatography, as a result of analyzing the amount of residual monomer in the denture base material manufactured by injection molding, the amount of residual monomer was higher in the self-curing denture base material than in the injection-molded or heat-cured denture base material [14]. Residual monomer was also revealed to cause growth inhibition of fibroblasts in the oral cavity [15], create inappropriate oral surface conditions [16], and lead to clinical hypersensitivity reactions [17,18].
- In this study, the amount of residual monomer was analyzed by UPLC by preparing a specimen according to ISO standards using a self-curing resin that has been traditionally used and a self-curing resin for CAD/CAM that has been used recently [19]. The cytotoxicity of the liquid polymerization resin was assessed in the second and third stages for the CAD/CAM and hand-mixed self-curing resins, and that of the solid polymerization resin was assessed in the fourth stage of polymerization.
- The amount of eluted residual monomer should be no more than 4.5 wt% according to the international standard; the CAD/CAM self-curing resin was eluted at 1.07 wt% and the hand-mixed self-curing resin was eluted at 4.48 wt%. This difference occurs because the CAD/CAM self-curing resin is manufactured by mechanical mixing and compression. Although the two products were within the international standard, the amount of residual monomer was higher in the hand-mixed self-curing resin than in the CAD/CAM self-curing resin, with a difference of approximately four times (Table 1).
- According to previous studies, explanted primary human cells such as human dental pulp cells and gingival and periodontal ligament fibroblasts have also been used in cytotoxicity tests [20,21], but primary cells have a limited lifespan and are more difficult to maintain and use [22]. Appropriate cell lines must be characterized to facilitate in vitro cytotoxicity studies. In the present study, L929 fibroblasts were used for cell culture. In the cytotoxicity evaluation, the mixing ratio of the exclusive powder and exclusive solution was 2:1 by weight; therefore, >30% of the mixture was exclusive solution. In this study, cell death was confirmed, except when ≤0.2% of the liquid resin polymer during polymerization was tested. In contrast, the solid resin polymer exhibited low cytotoxicity (Fig. 2). The ISO standards for solid resin polymers are evaluated based on the polymerization completion stage. Because the product is completely formed to the standards, it is natural to evaluate whether the cytotoxicity is within the standards, as in other studies of cytotoxicity in dentures installed in the mouth [23].
- There is a clear limitation in that cytotoxicity was tested using liquid resin at an arbitrary ratio instead of testing the polymerized resin, as in clinical situations. However, the above method was devised to standardize the effect on cells of the chemical substances from a chemical reaction step that occurs in a relatively short time.
- In this study, we confirmed that the liquid resin polymer has a harmful effect on the mucosal cells of the oral cavity, even at the second (stringy) and third (dough) stages, which is the entire range of polymerization completion and is outside the ISO standard. Therefore, the ISO standards for self-curing resins used directly in the oral cavity should have high requirements that consider stability not only in the finished product but also in the polymerization process. Owing to the characteristics of the material, it exhibits excellent color stability, workability, and final strength, and polishing is possible. However, heat is generated during polymerization in the oral cavity and unreacted residual monomers exude through the expansion of the resin substrate surface due to moisture [24,25]. The presence of high residual amounts of monomers is consistent with previous studies [26].
- After polymerization was complete, the residual monomer was tested according to the standard. As cytotoxicity was confirmed during the polymerization process, additional research was performed to examine and analyze the degree of residual monomer leakage over time from stages 2 and 3 of the polymerization process to the time when production was completed and attached to the oral cavity of the patient.
- The self-curing resin for provisional crown production not only serves as an intermediate step in treatment but also plays an important role in determining the prognosis after treatment [27]. In addition, it replaces hardened teeth that have been lost or prepared and prevents physical, chemical, and physiological problems in the prepared abutment [27]. Currently, marketability and productivity are increasing, reducing the manufacturing time and inconvenience of patient visits, and self-curing resin has the advantage of high accuracy for direct treatment in the oral cavity [28]. However, information on the harmfulness of residual monomers and their toxicity to the human body during mixed polymerization must be assessed to prevent them. Because cytotoxicity did not occur after polymerization, the crown would have to be manufactured indirectly from a dental model using a dental impression to safely use the product. In addition, it will be necessary to adjust the product standards of resin manufacturing companies for market performance and commercial growth, and provide oral health information to dentists, dental technicians, and dental hygienists to inform them of product usage standards.
- Within study limitations, residual monomer and cytotoxicity tests were performed to analyze the effect of the self-curing resin for temporary crown generation on the oral mucosa. The amount of residual monomer in both products fell within the ISO standard range; however, it was confirmed that the residual monomer from the manually mixed self-curing-type resin was more than four times higher than that from the self-curing-type resin for CAD/CAM. In the WST test, 73.4% of the cells survived 0.2% of the liquid resin polymer collected during polymerization. The average cell viability of the solid resin polymer was higher than the cell viability standard of 70% for both the CAD/CAM and hand-mixed self-curing resins. Depending on the manufacturing method and step-by-step polymerization process, temporary crowns can have different effects on the oral cavity. Since the second and third stages of the polymerization process of the hand-mixed self-curing-type resin can have harmful effects on the oral mucosa, crowns composed of this type of resin must be manufactured indirectly using a dental model. Because cytotoxicity is not evident in the solid resin polymer after polymerization, it is necessary to indirectly prepare a temporary crown on the dental model to safely handle the product.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
-
Author contributions
Conceptualization: all authors; Data curation, Visualization, Software: JK; Formal analysis, Methodology, Investigation, Supervision, Validation: JS, SK; Writing-original draft: JK, JS; Writing-review & editing: SK.
Notes
- 1. Magne P, Magne M, Belser U. The diagnostic template: a key element to the comprehensive esthetic treatment concept. Int J Periodontics Restorative Dent 1996;16:560–9.PubMed
- 2. Alpert RL. A method to record optimum anterior guidance for restorative dental treatment. J Prosthet Dent 1996;76:546–9.ArticlePubMed
- 3. Hernandez EP, Oshida Y, Platt JA, Andres CJ, Barco MT, Brown DT. Mechanical properties of four methylmethacrylate-based resins for provisional fixed restorations. Biomed Mater Eng 2004;14:107–22.PubMed
- 4. Kerby RE, Knobloch LA, Sharples S, Peregrina A. Mechanical properties of urethane and bis-acryl interim resin materials. J Prosthet Dent 2013;110:21–8.ArticlePubMed
- 5. Burns DR, Beck DA, Nelson SK; Committee on Research in Fixed Prosthodontics of the Academy of Fixed Prosthodontics. A review of selected dental literature on contemporary provisional fixed prosthodontic treatment: report of the Committee on Research in Fixed Prosthodontics of the Academy of Fixed Prosthodontics. J Prosthet Dent 2003;90:474–97.ArticlePubMed
- 6. Fondriest JF. Using provisional restorations to improve results in complex aesthetic restorative cases. Pract Proced Aesthet Dent 2006;18:217–23.PubMed
- 7. Priest G. Esthetic potential of single-implant provisional restorations: selection criteria of available alternatives. J Esthet Restor Dent 2006;18:326–38.ArticlePubMed
- 8. Staehle HJ, Sekundo C. The origins of acrylates and adhesive technologies in dentistry. J Adhes Dent 2021;23:397–406.ArticlePubMed
- 9. Harrison A, Huggett R. Effect of the curing cycle on residual monomer levels of acrylic resin denture base polymers. J Dent 1992;20:370–4.ArticlePubMed
- 10. Abdallah MN, Tran SD, Abughanam G, Laurenti M, Zuanazzi D, Mezour MA, et al. Biomaterial surface proteomic signature determines interaction with epithelial cells. Acta Biomater 2017;54:150–63.ArticlePubMed
- 11. Souto-Lopes M, Azevedo Á, Teixeira A, Bastos-Aires D, Lordelo J, Pérez-Mongiovi D. Cytotoxicity of acrylic-based resin compounds in a human gingival fibroblast cell line. Rev Port Estomatol Med Dent Cir Maxilofac 2013;54:87–90.Article
- 12. Wiegand A, Stucki L, Hoffmann R, Attin T, Stawarczyk B. Repairability of CAD/CAM high-density PMMA- and composite-based polymers. Clin Oral Investig 2015;19:2007–13.ArticlePubMedPDF
- 13. Herráez-Galindo C, Rizo-Gorrita M, Luna-Oliva I, Serrera-Figallo MÁ, Castillo-Oyagüe R, Torres-Lagares D. In vitro comparative study of fibroblastic behaviour on polymethacrylate (PMMA) and lithium disilicate polymer surfaces. Polymers (Basel) 2019;11:744.ArticlePubMedPMC
- 14. Lee HJ, Kim CW, Kim YS. The level of residual monomer in injection molded denture base materials. J Korean Acad Prosthodont 2003;41:360–8.
- 15. Kedjarune U, Charoenworaluk N, Koontongkaew S. Release of methyl methacrylate from heat-cured and autopolymerized resins: cytotoxicity testing related to residual monomer. Aust Dent J 1999;44:25–30.ArticlePubMed
- 16. Naji A, Harmand MF. Study of the effect of the surface state on the cytocompatibility of a Co-Cr alloy using human osteoblasts and fibroblasts. J Biomed Mater Res 1990;24:861–71.ArticlePubMed
- 17. Arossi GA, Lehmann M, Dihl RR, Reguly ML, de Andrade HH. Induced DNA damage by dental resin monomers in somatic cells. Basic Clin Pharmacol Toxicol 2010;106:124–9.ArticlePubMed
- 18. Gautam R, Singh RD, Sharma VP, Siddhartha R, Chand P, Kumar R. Biocompatibility of polymethylmethacrylate resins used in dentistry. J Biomed Mater Res B Appl Biomater 2012;100:1444–50.ArticlePubMed
- 19. Al-Hiyasat AS, Darmani H, Milhem MM. Cytotoxicity evaluation of dental resin composites and their flowable derivatives. Clin Oral Investig 2005;9:21–5.ArticlePubMedPDF
- 20. Kang S. Mineralization-inducing potentials of calcium silicate-based pulp capping materials in human dental pulp cells. Yeungnam Univ J Med 2020;37:217–25.ArticlePubMedPMCPDF
- 21. Lai YL, Chen YT, Lee SY, Shieh TM, Hung SL. Cytotoxic effects of dental resin liquids on primary gingival fibroblasts and periodontal ligament cells in vitro. J Oral Rehabil 2004;31:1165–72.ArticlePubMed
- 22. Freshney RI. Transformation and immortalization. In: Freshney RI, editors. Culture of animal cells: a manual of basic technique and specialized applications. 6th ed. New Jersey: Wiley-Blackwell; 2010. p. 279–97.
- 23. Chaves CA, Machado AL, Carlos IZ, Giampaolo ET, Pavarina AC, Vergani CE. Cytotoxicity of monomers, plasticizer and degradation by-products released from dental hard chairside reline resins. Dent Mater 2010;26:1017–23.ArticlePubMed
- 24. Göpferich A, Schedl L, Langer R. The precipitation of monomers during the erosion of a class of polyanhydrides. Polymer 1996;37:3861–9.Article
- 25. Jorge JH, Giampaolo ET, Machado AL, Vergani CE. Cytotoxicity of denture base acrylic resins: a literature review. J Prosthet Dent 2003;90:190–3.ArticlePubMed
- 26. Trivedi SC, Talim ST. The response of human gingiva to restorative materials. J Prosthet Dent 1973;29:73–80.ArticlePubMed
- 27. Pituru SM, Greabu M, Totan A, Imre M, Pantea M, Spinu T, et al. A review on the biocompatibility of PMMA-based dental materials for interim prosthetic restorations with a glimpse into their modern manufacturing techniques. Materials (Basel) 2020;13:2894.ArticlePubMedPMC
- 28. Kim EK, Park EY, Kang S. Three-dimensional printing of temporary crowns with polylactic acid polymer using the fused deposition modeling technique: a case series. J Yeungnam Med Sci 2023;40:302–7.Article
References
Figure & Data
References
Citations


 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine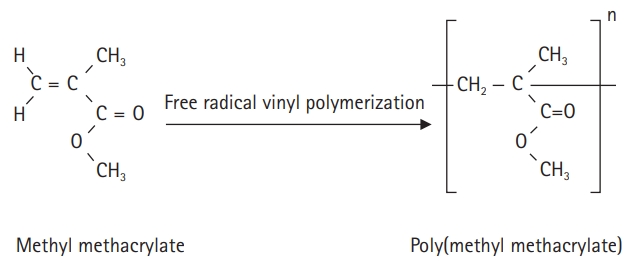

 PubReader
PubReader ePub Link
ePub Link Cite
Cite