Enhancing ketamine anesthesia with midazolam and fentanyl for children’s ear surgery: a prospective randomized study
Article information
Abstract
Background
Myringotomy with tympanostomy tube insertion (MTI) is a superficial surgical procedure used to prevent hearing loss in children with serous otitis media. Intravenous anesthesia, often ketamine, is preferred for this procedure because of its ability to induce sedation without compromising airway reflexes. However, ketamine alone may be insufficient and potentially lead to spontaneous movement during surgery. This study evaluated the effectiveness of midazolam and fentanyl as adjuvants to ketamine in reducing spontaneous movement during MTI and enhancing the quality of recovery.
Methods
This study involved two groups of 30 patients each: one group received intravenous ketamine (1.5 mg/kg) with an equal volume of normal saline (K group), while the other received a combination of midazolam, fentanyl, and ketamine (0.05 mg/kg, 1 μg/kg, and 1.5 mg/kg, respectively; MFK group). We assessed side effects, intraoperative patient movement, surgeon satisfaction, and emergence agitation scores.
Results
The MFK group exhibited significantly lower scores for patient movement (p<0.01) and emergence agitation (p<0.01) and markedly higher surgeon satisfaction scores (p<0.01) than the K group.
Conclusion
Administering a midazolam-fentanyl-ketamine combination effectively reduced spontaneous movement during surgery and emergence agitation during recovery without prolonging discharge times in children undergoing MTI.
Introduction
Otitis media with effusion (OME) is a common disease in children with colds, and untreated OME can result in hearing loss [1]. Myringotomy with tympanostomy tube insertion (MTI), a surgical treatment for OME, is one of the most common daycare surgeries performed in children. As MTI is a brief and superficial operative procedure, it requires only intravenous anesthesia with ketamine instead of general anesthesia with endotracheal intubation [2]. Ketamine is well known for achieving analgesia and sedation without affecting airway reflexes [3] and is regarded as a suitable agent for procedural sedation [4]. Administration of sub-dissociative doses of ketamine is recommended to reduce the incidence of side effects [5]. However, at clinical doses, ketamine often provides inadequate anesthesia [6]. In addition, a single movement of the patient can lead to MTI failure or dislodgement of the tympanostomy tube from the tympanic membrane. To prevent patient movement and provide adequate anesthesia during surgery, intravenous adjuvant therapy is recommended along with ketamine [7,8].
The combination of two other drugs, midazolam and fentanyl, has potential as an adjuvant therapy. The former is widely used for its amnestic and anxiolytic effects as well as its minimal adverse effects following delayed emergence from general anesthesia [9], while fentanyl is a commonly used, highly potent, short-acting narcotic [10]. Despite the reported role of fentanyl in preventing extreme agitation, it has major side effects such as respiratory depression, nausea, and vomiting [11].
The present prospective, randomized study suggests that the combination of low-dose midazolam and fentanyl as adjuvants to ketamine prevents spontaneous movement during MTI and improves the quality of recovery compared to ketamine anesthesia alone. Thus, this anesthetic strategy balances the disadvantages of the individual drugs.
Methods
Ethical statements: This study was approved by the Institutional Review Board (IRB) of The Catholic University of Korea, St. Vincent’s Hospital (IRB No: VC12MISI0113), and written informed consent was obtained from all study participants and their parents. This study was registered at CRIS (Clinical Research information Service; No. KCT0003721).
1. Patients and treatment
Sixty patients aged 1 year to 15 years with American Society of Anesthesiologists physical status classification I or II scheduled for elective bilateral MTI under intravenous anesthesia with ketamine were enrolled in this study.
Patients were assigned to two groups using computer-generated randomization; one group received intravenous ketamine (K group, n=30) and the other group received a combination regimen of midazolam, fentanyl, and ketamine (MFK group, n=30). Randomization was performed via a computer and maintained in an opaque envelope that was opened immediately before the study was initiated. None of the patients was premedicated. Immediately before surgery, standard monitors, including an electrocardiogram and a pulse oximeter, were connected and baseline values were recorded. All patients received 1.5 mg/kg intravenous ketamine. Patients in the MFK group were also administered 0.05 mg/kg midazolam and 1 μg/kg fentanyl [7,12]. Patients in the K group were administered an equal volume of normal saline instead of the midazolam/fentanyl. Anesthetic delivery was performed by a nurse who was blinded to the study process. The surgery was performed under adequate anesthesia. During the operation, 0.5 mg/kg ketamine was administered when the patients showed heavy breathing, moved their extremities or trunk, or developed singultus.
Demographic data, including age, sex, and weight, were recorded. The heart rate and arterial oxygen saturation were continuously monitored using an electrocardiogram and pulse oximeter. Designated investigators assessed the adequacy of anesthesia according to a four-step scale (patient movement score): 0=asleep, calm, and fully flaccid; 1=wiggling of the extremities, breathing harshly, or grimacing; 2=slight movement of the extremities or body but not the head; and 3=thrashing around, including movement of the head. Additional ketamine was administered to patients with scores of 2 to 3. Operating conditions were recorded according to a three-step scale by the surgeon (surgeon satisfaction score): 1=excellent conditions for surgery; 2=average conditions for surgery, overall good, but sometimes surgery was interrupted by unexpected movement; and 3=poor surgical conditions, difficult to operate safely due to interruption by frequent movement of the patient. The duration of anesthesia, dose of ketamine, need for airway assistance, or event of desaturation defined as an oxygen saturation <90% were also recorded. Recovery profiles, including recovery time (time interval from the end of surgery to eye opening and responding to verbal commands), discharge time (time interval from arrival in the post-anesthesia care unit [PACU] to patient discharge from the PACU), and quality of emergence, were recorded. Patients were discharged from the PACU when the Aldrete recovery score reached ≥9 [13].
The quality of emergence was evaluated using a four-point agitation/discomfort scale (emergence agitation score) [4]: 1=calm, 2=crying but can be consoled, 3=crying and cannot be consoled, and 4=agitated and thrashing around. Side effects, including dizziness, nausea, vomiting, and the need for oxygen therapy or airway assistance, were evaluated.
2. Statistical analysis
Sample size was determined using a power analysis to achieve an 80% chance (β=0.2) of detecting a 30% reduction in the incidence of side effects using midazolam and fentanyl with an assumed significance level of α=0.05, based on a previous outcome [4]. The sample size was calculated to be 30 patients per group. Statistical analyses were performed using SPSS ver. 18.0 for Windows (SPSS Inc., Chicago, IL, USA). Continuous variables were expressed as mean±standard deviation and compared using a Student t-test and one-way analysis of variance. Discrete variables (adequacy of anesthesia, operating conditions, quality of emergence, and side effects) were compared using the chi-square test. A p-value of 0.05 was considered statistically significant.
Results
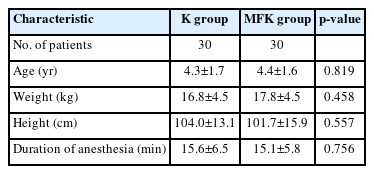
Sixty children were enrolled and randomly assigned to either the K (n=30) or MFK (n=30) group. Demographic data including age, weight, height, and duration of anesthesia were not significantly different between the two groups (Table 1).
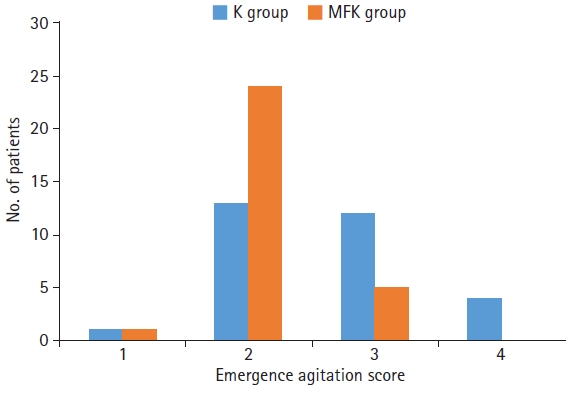
Regarding intraoperative characteristics, the MFK group had significantly lower patient movement scores (p<0.01) (Fig. 1) and better surgeon satisfaction scores (p<0.01) than the K group (Fig. 2).
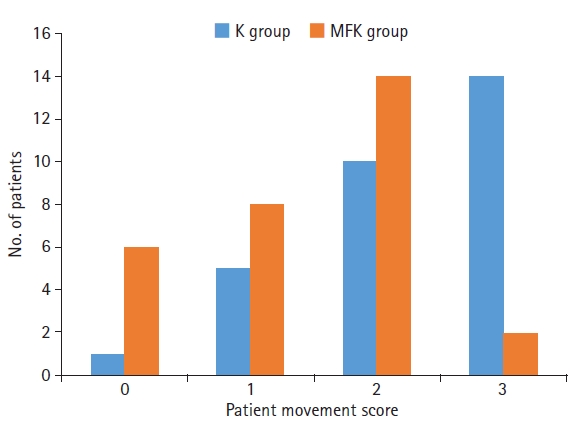
Intraoperative characteristics of the midazolam, fentanyl, and ketamine (MFK) group are marked by significantly lower patient movement scores than those of the ketamine (K) group (p<0.01); 0=asleep, calm, and fully flaccid; 1=wiggling of extremities, breathing harshly, or grimacing; 2=slight movement of extremities or body but not head; and 3=thrashing around, including head movement.
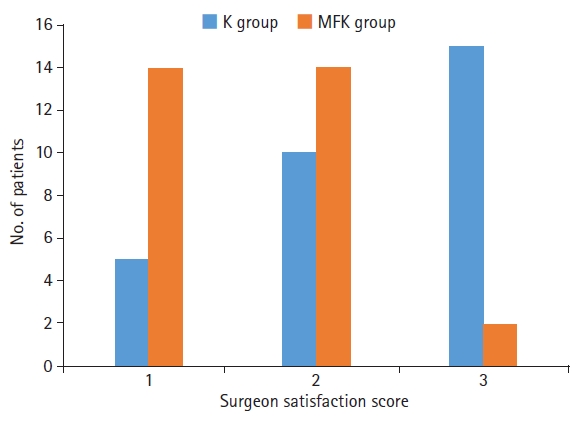
Intraoperative characteristics of the midazolam, fentanyl, and ketamine (MFK) group are marked by significantly better surgeon satisfaction scores than those of the ketamine (K) group (p<0.01); 1=excellent surgical conditions, 2=average surgical conditions, overall good but sometimes surgery was interrupted by unexpected movement, and 3=poor surgical conditions.
The mean patient movement score and surgeon satisfaction score were 1.42±0.83 and 1.58±0.61, respectively, in the MFK group and 2.29±0.84 and 2.38±0.74, respectively, in the K group.
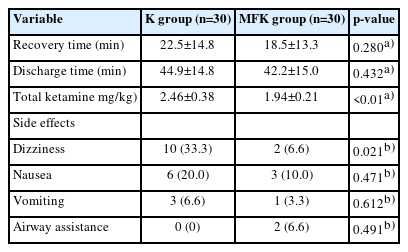
The calculated ketamine dose per weight for the maintenance of anesthesia was also significantly lower in the MFK group than in the K group (p<0.01) (Table 1). There were no significant differences between the two groups in terms of recovery and discharge times (Table 2).
However, a significantly lower emergence agitation score was observed in the MFK group than in the K group (p<0.01) (Fig. 3). Except for dizziness, no statistically significant differences were found between the two groups with respect to side effects, including nausea, vomiting, and the need for airway assistance (Table 2).
Discussion
The present study demonstrated that a combined regimen of intravenous fentanyl and midazolam as adjuvants to ketamine attenuates intraoperative patient movement and emergence agitation in children undergoing MTI more effectively than ketamine alone.
MTI is frequently performed in children with OME to improve hearing. Surgery of the middle ear requires care and sophistication on account of the narrowness of the external auditory meatus and the small size of the tympanostomy tube; a slight movement of the patient can displace the tympanostomy tube or damage adjacent tissues. Therefore, most otolaryngologists require patients to be fully paralyzed during surgery. Nonetheless, the use of inhalational anesthetics with non-depolarizing muscle relaxants is unnecessary during MTI because myringotomy is an uncomplicated procedure involving only a minimal incision. Although it can cause relatively severe pain, even a bilateral MTI takes less than 20 minutes to perform. Muscle relaxants are usually avoided when administering maintenance anesthesia for brief superficial surgery because of the associated side effects and postoperative residual paralysis that they induce. Furthermore, insufficient time to reach the target concentration of inhalational anesthetics may result in inadequate anesthesia. The risks associated with inhalational anesthetics—upper respiratory tract infection, laryngospasm, emergence agitation in children, the consequent prolonged hospital stays, and dissatisfaction among patients and parents—are thus often taken in vain [14,15].
For decades, ketamine has been widely used to facilitate deep sedation, analgesia, and amnesia. The beneficial properties of this phencyclidine derivative include preservation of the airway reflex, limiting cardiovascular and respiratory side effects, and established safety [16-19]. However, in clinical use, repeated doses of ketamine are often administered to patients to prevent inadequate anesthesia or prolong the effect of sedation. Theoretically, there is no need to exceed the dissociative dose of ketamine because higher doses do not enhance sedation. If preventing spontaneous movement is a priority, propofol is more effective than ketamine alone [20]. Additional doses of ketamine can lead to several side effects such as increased sympathetic tone, excessive salivation, emesis, and laryngospasm [21]. Therefore, ketamine doses >2 mg/kg are not recommended [22]. In addition to the side effects of ketamine, its association with emergence reactions limits its use for procedural sedation. The overall incidence of ketamine-induced emergence reactions varies from 5% to 15%, while another study reported that 1.6% of pediatric patients experience clinically significant agitation [23,24]. In the present study, 13.3% of the patients (n=4) in the K group manifested severe emergence agitation (emergence agitation score of 4). This finding is consistent with most previous data but exceeded the results of Green et al. [25], which may be attributed to the difficulty in distinguishing early emergence delirium from pain behavior in preschool children.
To attenuate the side effects of ketamine while maintaining an adequate anxiolytic effect, midazolam is frequently used as an adjuvant. Sener et al. [26] demonstrated the role of midazolam and ketamine co-administration in decreasing the effect of the latter on emergence agitation, which is consistent with our results. However, the addition of midazolam as an adjuvant has a limited effect on reducing emergence reactions in children [27]. The co-administration of midazolam and ketamine further results in delayed awakening from anesthesia, which is problematic considering that MTI is a daycare surgery and demands quick recovery for safe discharge. Thus, to reduce emergence reactions following ketamine injection and shorten delayed awakening, we used a smaller dose of midazolam than that used in previous studies [4,28].
Finkel et al. [28] reported that premedication with a 2 μg/kg dose of fentanyl significantly decreased agitation levels without affecting recovery or discharge times in patients undergoing bilateral myringotomy. Our findings also demonstrate the effectiveness of low-dose fentanyl in conjunction with a midazolam-ketamine regimen in reducing patient movement and emergence agitation without delaying recovery.
The combined midazolam-fentanyl-ketamine regimen was additionally assessed in terms of postoperative pain, which may not only induce perioperative patient movement but also emergence agitation [14]; appropriate postoperative analgesia presents an effective means of preventing emergence agitation [28,29]. Our results showed a 68.8% lower incidence of moderate (score of 3) and severe (score of 4) emergence agitation in the MFK group than in the K group, suggesting that fentanyl treatment helps to attenuate emergence agitation.
Ketamine is further associated with random involuntary movements of the head or extremities unrelated to painful stimuli [30]. The present study found that the MFK group had improved patient and surgeon satisfaction scores compared with those of the K group. We attribute this difference to both the lower dose of ketamine administered to the MFK group and the benefits of the two adjuvant drugs.
The most common neurological side effects of ketamine include headaches, dizziness, blurred vision, and nausea [31]. The overall incidence of dizziness observed in previous studies ranged from 22% to 44% according to the methodology used [31,32], which is consistent with our findings in the K group. The decrease in dizziness incidence for the MFK group was 26.7% higher than that for the K group, likely due to the lower dose of ketamine. Referring to the opioid-sparing effects of ketamine, a previous study investigated whether low-dose ketamine might yield an antiemetic effect; however, the findings were not significant [33].
In the present study, the incidence of nausea and vomiting did not differ significantly between the two groups. Exposure to ketamine usually provides adequate maintenance of the airway muscles. Although two patients in the MFK group required oxygen for airway assistance during recovery, the incidence of adverse respiratory events was not significantly different between the two groups. This finding suggests that co-administration of low-dose midazolam and fentanyl is associated with minimal respiratory depression.
This study demonstrated that the co-administration of midazolam and fentanyl as adjuvants to ketamine effectively reduced patient movement and emergence agitation scores without delaying the recovery or discharge of pediatric patients. Furthermore, the surgeon satisfaction score was significantly higher in the MFK group than in the K group, indicating that the former provided better operating conditions than the latter. No evidence of adverse effects, including nausea, vomiting, and the need for airway assistance, was observed. Furthermore, dizziness was less likely to occur in the MFK group than in the K group.
In conclusion, the combination of low-dose midazolam and fentanyl with ketamine may be superior to ketamine alone in preventing the spontaneous movement of patients during surgery and reducing emergence agitation. If implemented, this anesthetic regimen would improve the quality of anesthesia and recovery in pediatric patients undergoing MTI.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: all authors; Data curation: SMH, SYK, JHI; Formal analysis: JDJ, SYK; Methodology: SYK; Project administration, Software: JHI; Visualization, Investigation: SMH; Supervision: JDJ; Writing-original draft: SMH; Writing-review & editing: JDJ.