Oncological and functional outcomes following robot-assisted laparoscopic radical prostatectomy at a single institution: a minimum 5-year follow-up
Article information
Abstract
Background
To evaluate mid-term oncological and functional outcomes in patients with prostate cancer treated by robot-assisted laparoscopic radical prostatectomy (RALP) at our institution.
Methods
We retrospectively reviewed the medical records of 128 patients with prostate cancer who underwent RALP at our institution between February 2008 and April 2010. All patients enrolled in this study were followed up for at least 5 years. We analyzed biochemical recurrence (BCR)-free survival using a Kaplan-Meier survival curve analysis and predictive factors for BCR using multivariate Cox regression analysis. Continence recovery rate, defined as no use of urinary pads, was also evaluated.
Results
Based on the D’Amico risk classification, there were 30 low-risk patients (23.4%), 47 intermediate-risk patients (38.8%), and 51 high-risk patients (39.8%), preoperatively. Based on pathological findings, 50.0% of patients (64/128) showed non-organ confined disease (≥T3a) and 26.6% (34/128) had high grade disease (Gleason score ≥8). During a median follow-up period of 71 months (range, 66-78 months), the frequency of BCR was 33.6% (43/128) and the median BCR-free survival was 65.9 (0.4-88.0) months. Multivariate Cox regression analysis revealed that high grade disease (Gleason score ≥8) was an independent predictor for BCR (hazard ratio=4.180, 95% confidence interval=1.02-17.12, p=0.047). In addition, a majority of patients remained continent following the RALP procedure, without the need for additional intervention for post-prostatectomy incontinence.
Conclusion
Our study demonstrated acceptable outcomes following an initial RALP procedure, despite 50% of the patients investigated demonstrating high-risk features associated with non-organ confined disease.
INTRODUCTION
Prostate cancer (PCa) is the second most commonly diagnosed cancer in the world, behind only lung cancer. It is the most common cancer in men, accounting for 25% of all cancers affecting men. The increased incidence of PCa has resulted primarily from improved prostate specific antigen (PSA) screening, which can detect many early-stage PCa cases [1]. In Korea, PCa incidence doubled between 2007 and 2013, and the PCa mortality rate increased slightly [2]. A variety of treatment options are available for the management of localized PCa. Radical prostatectomy is a common treatment modality, which can be performed via an open, laparoscopic, or robot-assisted laparoscopic approach [3,4]. Although several reports have described short-term oncological and functional outcomes associated with robot-assisted laparoscopic radical prostatectomy (RALP) procedures, few studies have described outcomes after a sufficiently long follow-up period. As highlighted by a previous formal systematic review, there is no solid body of evidence establishing the superiority of any one surgical approach over the others. Some studies has shown that RALP has been increasingly adopted worldwide as a surgical treatment option for localized PCa because of its minimally invasive nature and excellent oncological and functional outcomes following the procedure [5]. RALP is associated with lower perioperative morbidity and a lower rate of positive surgical margins, compared to a laparoscopic prostatectomy, although it is also associated with considerable methodological uncertainty. Some studies have reported an earlier recovery of urinary continence and erectile function in patients undergoing RALP compared to a contemporary series involving patients who underwent other prostatectomy procedures [6]. However, most studies have suggested that no formal differences in the occurrence of cancer-related continence or erectile dysfunction outcomes are present [7]. We evaluated mid-term oncological and functional outcomes in patients with PCa who underwent RALP at our institution, using at least 5 years of follow-up for assessment.
MATERIALS AND METHODS
1. Patients
The da Vinci Surgical System(Intuitive Surgical, Sunnyvale, CA, USA) was introduced at our institution in 2008. We reviewed the records of the initial 128 patients who underwent RALP between February 2008 and April 2010. All patients were available for at least 5 years of follow-up.
Our technique for RALP is as follows: a 4-arm robot is used and a total of six ports are placed. Patients were placed on the operating table in the standard 30º Trendelenburg position. Following the development of the Retzius space, we performed standard lymph node dissection (lateral limit, genitofemoral nerve; cephalad limit, bifurcation of the common iliac artery; caudal limit, endopelvic fascia; medial limit, bladder) and the preprostatic fat was removed. The bladder and prostate border were dissected first and then the bladder and prostate were divided using a Bovie knife along the bladder-prostate imaginary borderline, until the prostatic urethra was exposed. The prostatic urethra was then incised and a previously placed Foley urinary catheter was observed, before continuing the division of the bladder and prostate. When the prostate was completely divided, the seminal vesicles and vas deferens were exposed and divided, and the vascular structures around them were ligated. After opening Denonvilliers’ fascia, periprostatic tissue was then dissected in an antegrade fashion on each side, with scissors. Neurovascular bundles on both sides of the prostate gland were protected using an interfascial technique. With cephalad traction on the prostate, the urethra and the recto-urethralis muscle were then divided, close to the prostate. After posterior reconstruction (Rocco stitch), an urethrovesical anastomosis is performed according to the standard method. Adjuvant androgen deprivation therapy (ADT) was initiated in patients with a high probability of lymph node and distant organ metastases, with lymphovascular invasion (LVI) or margin positive 2 weeks after surgery [8].
Biochemical recurrence (BCR) was defined as an elevated PSA ≥0.2 ng/mL at least 6 weeks after surgery. The D’Amico classification, which evaluates the risk of recurrence following localized treatment of PCa categorizes patients into three groups based on blood PSA levels, Gleason scores, and tumor stages [4].
2. Statistical analyses
All data were collected based on a protocol approved by the Institutional Review Board. Analyzed variables included pathological parameters such as LVI and secondary treatments (androgen deprivation therapy or radiotherapy). Continuous variables assessed were median follow-up (in months), age of patient, body mass index (BMI), PSA, and prostate volume, measured using a transrectal ultrasound (TRUS). Categorical variables assessed were the clinical stage of disease, biopsy-related variables and the surgical Gleason score (GS), pathological stage of disease, LVI, and surgical margin status. The surgical GS was categorized as ≤6, 7, or ≥8. BCR-free survival rates were analyzed using Kaplan-Meier survival curve and Cox regression analysis. All statistical evaluations were performed using SPSS version 18.0 for Windows (SPSS Inc., Chicago, IL, USA), and a p-value <0.05 (two-tailed) was considered statistically significant. The Chi-square test was used to determine the difference in proportions for categorical variables, while the Student t-test was used to assess continuous variables.
RESULTS
Preoperative characteristics of patients are shown in Table 1. The mean age at RALP was 68.1±7.5 years, and the median follow-up period was 71 months (range, 66-78 months). The mean preoperative PSA was 16.0±14.8 ng/mL and the mean prostate volume was 36.6±16.1 cc. Based on the D’Amico risk classification, patients were separated into three groups: low-, intermediate-, and high-risk categories with 30 (23.4%), 47 (38.8%), and 51 (39.8%) members, respectively.
Postoperative pathological findings and follow-up results are shown in Table 2. Organ-confined disease (T2), extraprostatic extension (T3a), and seminal vesicle invasion (T3b) were present in 64 (50.0%), 42 (32.8%), and 22 (17.2%) patients, respectively. Pathologic Gleason scores of 6, 7, and 8-10 were detected in 24 (18.8%), 70 (54.7%), and 34 (26.6%) patients, respectively. Pathological stage N1 disease was detected in 3 (2.3%) patients. Positive surgical margin was observed in 65 patients (50.8%), and LVI was detected in 16 (12.5%) patients. The overall BCR rate was 33.6%(43/128 patients), and median BCR-free survival was 65.9 months (range, 0.4-88.0 months) (Fig. 1, Table 2). Adjuvant ADT following RALP was administered to 58 patients (45.3%). Among all patients, four patients underwent salvage radiotherapy and four patients received chemotherapy due to the development of castration resistant prostate cancer. We found that three (2.3%) patients died during the follow-up period; two of these deaths were determined to be disease-related.
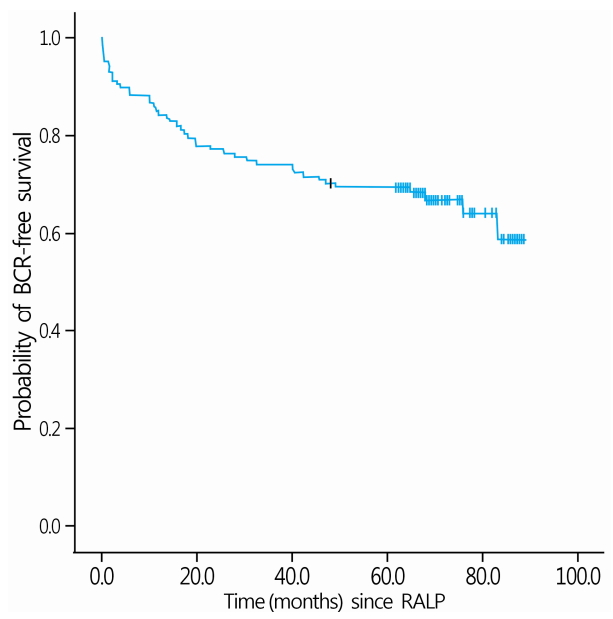
Kaplan-Meier survival curve analysis of biochemical recurrence-free survival for all patients. BCR, biochemical recurrence; RALP, robot-assisted laparoscopic radical prostatectomy.
Univariate analysis demonstrated that the high-risk category based on the D’Amico classification (p=0.003), elevated preoperative PSA (p=0.013), pathologic T stage (p=0.001), LVI (p=0.041), and GS ≥8 (p=0.005) were significantly associated with an increased risk of developing BCR(Table 3). Age, BMI, and prostate volume were not associated with BCR. Multivariate Cox regression analysis showed that a GS ≥8 was the only independent predictor of BCR (hazard ration [HR]=4.180, 95% confidence interval [CI]=1.02-17.12, p=0.047) (Table 4).
BCR-free survival periods based on the preoperative D’Amico risk classification were 76.5 months, 67.5 months, and 50.7 months, for the low-, intermediate-, and high-risk groups, respectively (p=0.001, Fig. 2A). Stratifying patients based on the pathological stage of disease showed that BCR-free survival periods were 70.8 months for organ-confined disease (T2), 66.3 months for T3a stage disease, and 38.7 months for T3b (p<0.001, Fig. 2B). BCR-free survival periods, stratified by the Gleason score showed 79.8 months, 66.5 months, and 50.0 months for a GS ≤6, 7, or ≥8, respectively (p=0.001, Fig. 2C). The presence of LVI also showed a statistically significant difference in terms of BCR-free survival rates (LVI (-), 66.8 months; LVI (+) 46.7 months; p=0.036) (Fig. 2D).
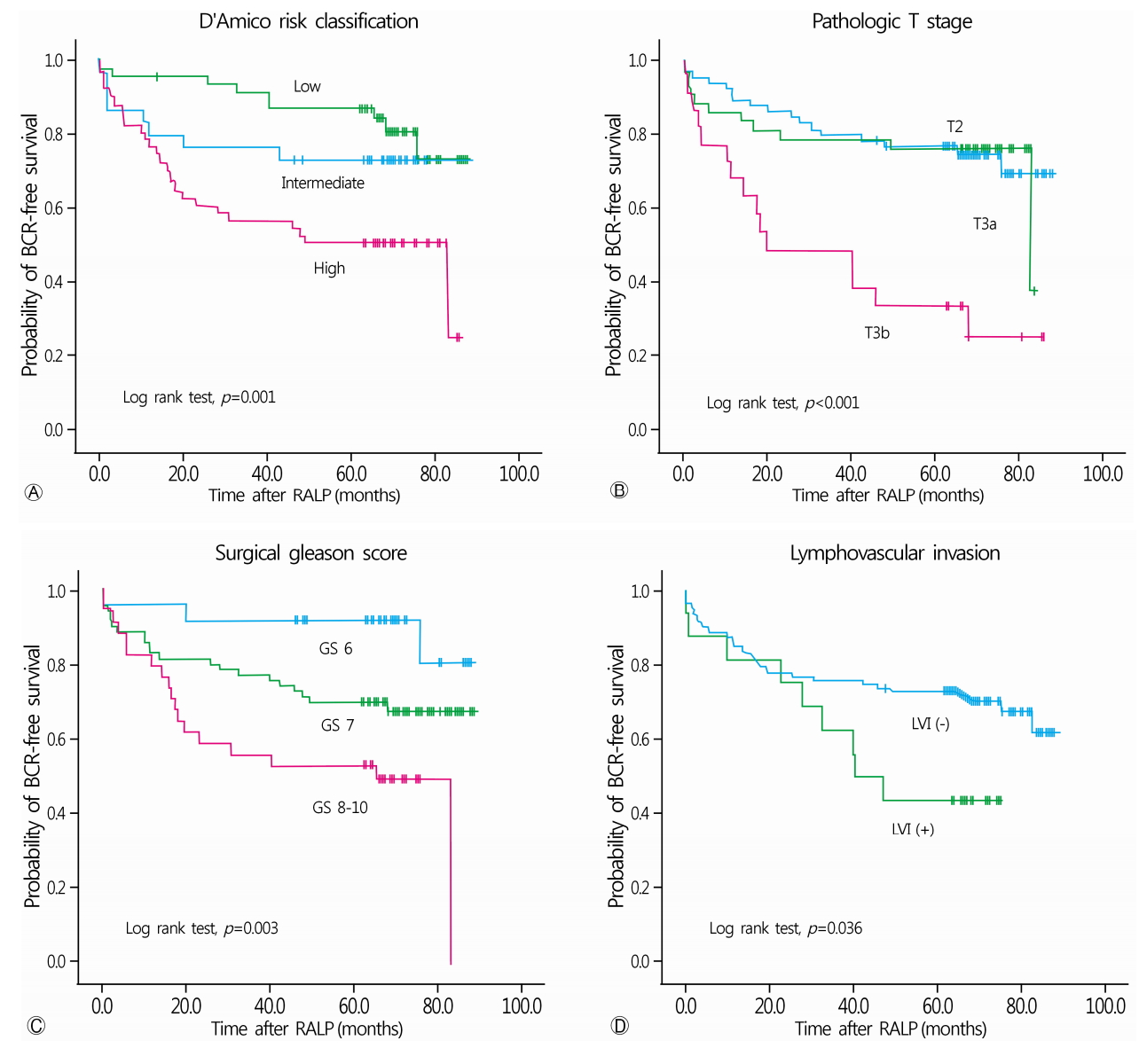
Kaplan-Meier survival curve analysis of biochemical recurrence-free survival comparing the three D’Amico risk groups (A), patients based on pathological staging (B), Gleason score (C), and Lymphovascular invasion (D), using the log-rank test. RALP, robot-assisted laparoscopic radical prostatectomy.
At the 1-year postoperative follow-up, we found that 33/128 (25.8%) patients experienced incontinence. However, 30 of 33 patients with urinary incontinence required only safety pads while the remaining three required additional intervention such as artificial urethral sphincter (AUR) implantation, for significant post-prostatectomy incontinence. Based on univariate analysis, a statistically significant difference was noted in terms of age between the continence and incontinence groups, however, no such statistically significant differences were observed using multivariate analysis (Table 5).
DISCUSSION
In our study, we analyzed mid-term oncological and functional outcomes in patients with PCa who underwent RALP at our institution. Fifty-one (39.8%) patients were classified as belonging to the high-risk group on preoperative D'Amico risk stratification. Postoperative pathologic results showed 34 (26.6%) patients with GS ≥8 and 64 (50%) of patients with T3, suggesting a relatively high ratio of high-risk patients. BCR was found in 43 (33.6%) patients and the median BCR-free survival period was 65.9 (0.4-88.0) months. The presence of high grade disease (Gleason score ≥8) was an independent factor affecting BCR development. At 1-year follow-up, urinary incontinence occurred in 33 (25.8%) patients, but only three patients required AUS. There were no factors influencing post-operative urinary incontinence.
In several studies of 400 to 1,384 men treated by RALP, the median time to BCR was 2.3-2.9 years. BCR-free survival rates were 74.0-87.1% and 81.0-84.5%, at 5 and 7 years [9-11]. According to BCR-free survival rate stratified by the D’Amico risk group in several studies, BCR-free survival rates were 96.8%, 95.1% and 92-92.6% in low-risk patients; 86.7%, 80.2% and 69.8-75% in intermediate-risk patients; and 78.2%, 72%, and 64-67.5% in high-risk patients at 3, 5, and 7 years after RALP, respectively [9,11,12]. In an Asian study, Abdel Raheem et al. [13] investigated 800 patients who underwent RALP with a median 64 months of follow-up. They revealed that BCR rates at 5-year follow-up were 54%, 45% and 58.1% for a high-risk D’Amico, GS ≥8, and clinical disease stage ≥cT2c, respectively. In our study, BCR-free survival rates were 71, 69.4, and 67.0% at 40-, 60-, and 80-months follow-up, respectively. Based on assessment of patients using the D’Amico risk classification, the 5-year BCR-free survival rate was 87.0% for the low-risk, 73.3% for the intermediate-risk, and 51.0% for the high-risk groups. According to pathologic T stage and GS, 5-year BCR-free survival rate were 76.2% for pT2 stage cancers, 76.5% for pT3a stage cancers, and 34.1% for T3b stage cancers and 91.7% for GS6, 67.5% for GS7, and 49.2% for GS 8-10. Overall BCR rate in our study was higher than that in other studies. We suggest that the higher BCR rate in this study was due to the initial experience performed with the first introduced robot system, and a relatively high proportion of high-risk group patients.
There are several views on the predictive factors of BCR when reviewing several studies. Rajan et al. [10] showed that preoperative PSA, GS, cT stage, pT stage, and >3mm multifocal PSMs were predictive factors of BCR. Lee et al. [12] showed that cT stage, pT stage, and pathological GS were independent predictive factors of BCR. Diaz et al. [14] suggested that D’Amico risk groups, pathologic GS, pathologic stage, and surgical margin status were predictive factors. Sooriakumaran et al. [11] revealed that preoperative PSA, pathologic GS, pathologic stage, surgical margin status and reduced surgeon volume were predictive factors. In this study, we observed that the high-grade disease (GS ≥8) was a predictive factor affecting BCR.
With respect to functional outcomes, a recent study showed that 366 men (21.3%) were incontinent 12 months after RALP [7]. Another study observed that 114 (63.3%) patients had post-prostatectomy incontinence (PPI) 1 month after RALP, and PPI persisted in 19 (16.0%) patients at 24 months [15]. The PPI rate in our study seems to be similar with other studies, despite the result based on early experiences of RALP. The factors affecting incontinence after surgery remain controversial. Some studies showed that the length of the membranous urethra and anatomical grades of nerve sparing can be risk factors associated with incontinence after RALP [16,17]. Another study [15] revealed that older age and longer operative time were highly relevant to short- and long-term PPI occurrence after RALP. In this retrospective study, we did not identify any significant factor for PPI.
This study has several limitations. First, our study was a retrospective and not a randomized, case-controlled study. Thus, detailed analysis was not performed for patient characteristics such as preoperative voiding patterns. Second, our data were based on results of surgery performed when the surgeon was uncomfortable with robot system, so the oncological outcomes of surgery might not be completely valid as they could represent results obtained during the surgeons’ learning curves. Third, our data did not provide information regarding patients’ potency and whether or not a nerve-sparing procedure had been performed. Therefore, we could not effectively analyze and comment upon the restoration of postoperative erectile function and effect of nerve-sparing procedures on postoperative continence or BCR-free survival rates. Fourth, we did not use a questionnaire to conduct patient interviews. Therefore, we subjectively divided patients into continence and incontinence groups based on chart reviews.
In conclusion, our study demonstrated acceptable outcomes following an initial RALP procedure, although 50% of the patients investigated demonstrated high-risk features associated with non-organ confined disease.
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Science, ICT & Future Planning (2014M3A9D3034164) (2014R1A1A3049460). Additional funding was provided by the Korean government (MSIP) (2015R1C1A1A01053509) (2016R1C1B1011180) and the Ministry of Education (2015R1D1A3A03020378).
Notes
No potential conflict of interest relevant to this article was reported.





