Can antioxidants be effective therapeutics for type 2 diabetes?
Article information
Abstract
The global obesity epidemic and the growing elderly population largely contribute to the increasing incidence of type 2 diabetes. Insulin resistance acts as a critical link between the present obesity pandemic and type 2 diabetes. Naturally occurring reactive oxygen species (ROS) regulate intracellular signaling and are kept in balance by the antioxidant system. However, the imbalance between ROS production and antioxidant capacity causes ROS accumulation and induces oxidative stress. Oxidative stress interrupts insulin-mediated intracellular signaling pathways, as supported by studies involving genetic modification of antioxidant enzymes in experimental rodents. In addition, a close association between oxidative stress and insulin resistance has been reported in numerous human studies. However, the controversial results with the use of antioxidants in type 2 diabetes raise the question of whether oxidative stress plays a critical role in insulin resistance. In this review article, we discuss the relevance of oxidative stress to insulin resistance based on genetically modified animal models and human trials.
Introduction
Although reactive oxygen species (ROS) are produced as a byproduct of oxygen metabolism, they play a significant role in normal cellular functions [1,2]. A well-organized antioxidant system maintains the physiological levels of ROS [3]. However, an imbalance between ROS production and antioxidant capacity causes ROS accumulation, which induces chemical modifications of DNA, protein, and lipids, leading to cellular damage, known as oxidative stress [1-3].
Oxidative stress is closely linked to a variety of diseases, including type 2 diabetes [1]. Type 2 diabetes is increasing globally due to the obesity pandemic and the growth of the aging population, and insulin resistance is a critical link between these two. Insulin resistance is known to be a critical risk factor for type 2 diabetes and other chronic diseases, such as cardiovascular diseases and cancers [4]. It is known that hyperglycemia in diabetic patients leads to serious complications by enhancing oxidative stress in the heart, kidney, and eyes [2]. Recently, oxidative stress has also been suggested to be a cause of insulin resistance [3,5]. Oxidative stress is increased in the plasma and tissue of patients and experimental animals with type 2 diabetes. Genetic modulation of antioxidant enzymes in rodents also supports the causative role of oxidative stress in insulin resistance. However, the inconsistent effects of antioxidant treatment on type 2 diabetes raise the question of whether oxidative stress induces insulin resistance. Thus, we sought to identify the role of oxidative stress in the development of insulin resistance based on animal experiments and human trials.
Reactive oxygen species and antioxidant systems
ROS are defined as oxygen-containing reactive species and include superoxide anion, hydrogen peroxide, hydroxyl radical, peroxynitrite, hypochlorous acid, and singlet oxygen [6]. The mitochondria electron transport chain, cell membrane nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase, cytochrome p450, and xanthine oxidase are the main intracellular sites of ROS generation, and among them, mitochondria is the primary source of ROS [7].
In the mitochondria, the generated superoxide anion is converted into hydrogen peroxide by superoxide dismutase 2 (SOD2). Subsequently, hydrogen peroxide is detoxified to form water in the presence of antioxidant enzymes such as glutathione peroxidase (GPx) or peroxidase or is exported to the cytoplasm (Fig. 1). Superoxide anions are also generated in the cell membrane and cytoplasm by NADPH oxidase and xanthine oxidase, respectively. In the cytoplasm, superoxide dismutase 1 (SOD1) catalyzes the conversion of superoxide anion to hydrogen peroxide, which is then detoxified to form water by enzymatic or nonenzymatic antioxidants, including glutathione, GPx, catalase, peroxiredoxin, and thioredoxin. In addition, catalase is a primary antioxidant that catalyzes hydrogen peroxide formation in the peroxisome. Oxidized methionine is reduced by methionine sulfoxide reductase in the cytoplasm, mitochondria, and endoplasmic reticulum. Moreover, peroxynitrite, produced by the reaction between nitric oxide and superoxide anion, and responsible for a wide array of tissue damage, is decomposed by peroxiredoxin, glutathione, and GPx [8,9].
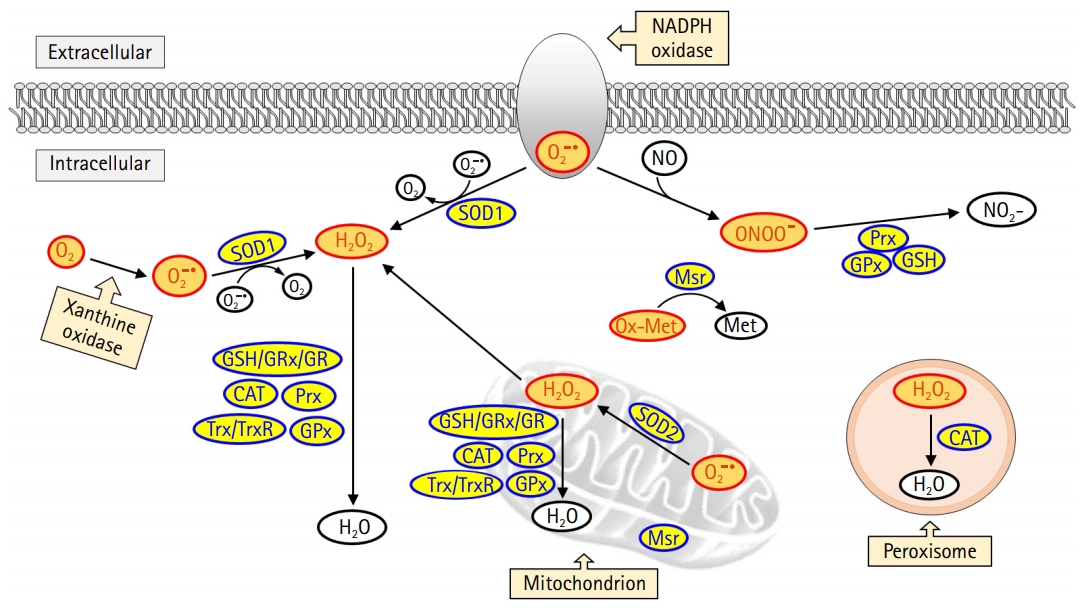
Intracellular reactive oxygen species (ROS) generation and the antioxidant scavenging system. ROS is produced from mitochondria, peroxisome, nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase, and xanthine oxidase. Among these sources, the mitochondrial electron transport chain is the primary source for ROS production. Superoxide dismutase 2 (SOD2) catalyzes the conversion of superoxide anion (O2–) into hydrogen peroxide (H2O2) in the mitochondria. The H2O2 is then detoxified in the mitochondria or moves to the cytoplasm. Cytoplasmic superoxide anion is generated from NADPH oxidase and xanthine oxidase, and subsequently converted into H2O2 by superoxide dismutase 1 (SOD1). H2O2 is detoxified by glutathione (GSH)/glutaredoxin (GRx)/glutathione reductase (GR), catalase (CAT), peroxiredoxin (Prx), thioredoxin (Trx)/thioredoxin reductase (TrxR), and glutathione peroxidase (GPx). Oxidized methionine (Ox-Met) is reduced by methionine sulfoxide reductase (Msr) to methionine (Met). Nitric oxide (NO) reacts with superoxide anion to form peroxynitrite (ONOO–), which is detoxified by Prx, GSH, and GPx.
Even though the intracellular antioxidant system is well developed to prevent ROS accumulation, ROS production can overwhelm the antioxidant capacity and induce oxidative damage to DNA, protein, and lipids.
Insulin signaling pathways
An increase in blood glucose levels after a meal induces insulin secretion from pancreatic β-cells, which leads to glucose uptake in skeletal muscle and adipose tissue by activating intracellular insulin signaling pathways. In the liver, insulin suppresses glycogen breakdown and gluconeogenesis while increasing glucose oxidation and glycogen synthesis. Inhibition of insulin signaling pathways in these peripheral tissues results in the development of insulin resistance. In this review, we will focus on insulin resistance in skeletal muscle (Fig. 2).
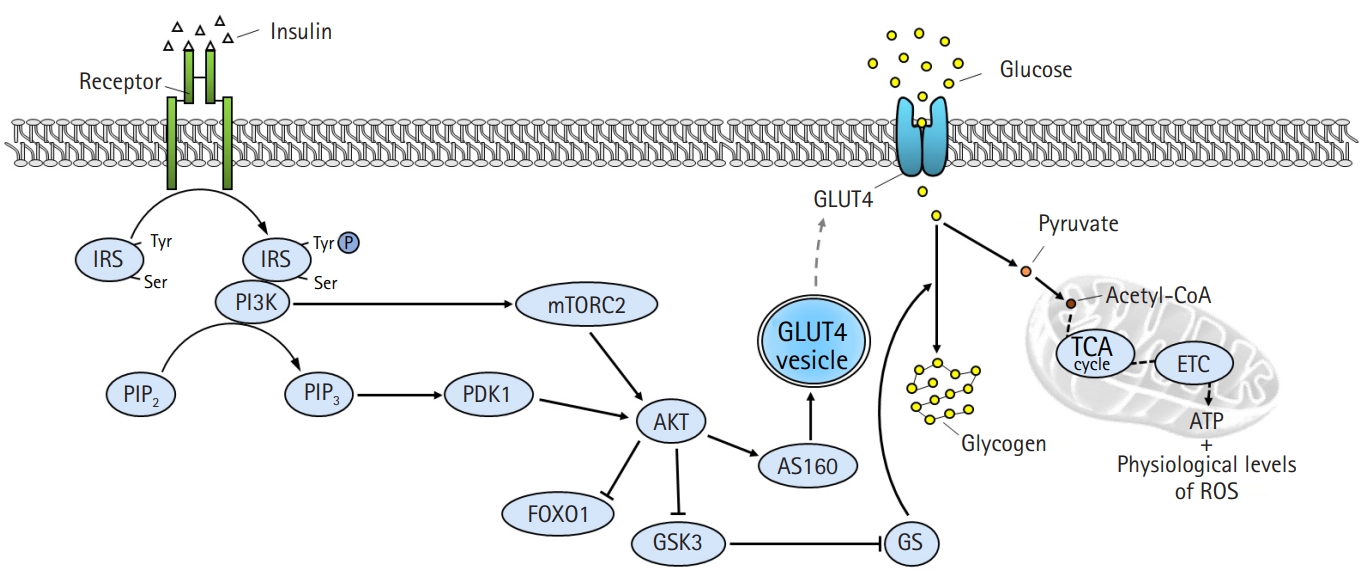
Intracellular insulin signaling pathway in skeletal muscle. Binding of insulin to insulin receptors (IR) on the plasma membrane promotes tyrosine autophosphorylation at the IR, which in turn induces tyrosine phosphorylation of the IR substrate (IRS). IRS activates the downstream substrate phosphatidylinositol 3-kinases (PI3K)/protein kinase B (AKT) pathway, and the activated AKT leads to increased glucose uptake and glycogen synthesis by inducing phosphorylation of AKT substrate of 160 kDa (AS160) and glycogen synthase kinase 3 (GSK3), respectively. Activated AS160 increases glucose uptake by mediating the translocation of glucose transporter type 4 (GLUT4) from the cytoplasm to the plasma membrane. Intracellular glucose is used for adenosine triphosphate (ATP) generation and glycogen synthesis. Tyr, tyrosine; Ser, serine; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-triphosphate; PDK1, phosphatidylinositide-dependent protein kinase 1; mTORC2, mammalian target of rapamycin complex 2; FOXO1, forkhead box protein O1; GS, glycogen synthase; acetyl-CoA, acetyl coenzyme A; TCA, tricarboxylic acid cycle; ETC, electron transport chain; ROS, reactive oxygen species.
Secreted insulin binds to insulin receptors on the plasma membrane and promotes autophosphorylation of tyrosine residues in the beta-subunit of the insulin receptor [10]. The activation of the insulin receptor immediately induces tyrosine phosphorylation of the insulin receptor substrate (IRS) proteins, which are initial downstream substrates of insulin signaling [11]. Phosphorylated IRS contains binding sites for numerous signal transduction partners with Src homology 2 domains, such as phosphatidylinositol 3-kinases (PI3K) [12]. Activated PI3K generates phosphatidylinositol (3,4,5)-triphosphate (PIP3) by catalyzing the phosphorylation of phosphatidylinositol 4,5-bisphosphate [13]. PIP3 induces the activation of protein kinase B (AKT), which is a key divergence point in the insulin signaling pathway [14]. Activation of AKT is induced via phosphorylation of threonine and serine residues by phosphatidylinositide-dependent protein kinase 1 and mammalian target of rapamycin complex 2 [15]. Subsequently, activated AKT phosphorylates numerous downstream substrates, including forkhead box protein O1 (FOXO1), AKT substrate of 160 kDa (AS160), and glycogen synthase kinase 3 (GSK3) [16]. The phosphorylation of GSK3 releases the inhibitory effect of GSK3 on glycogen synthase, leading to increased glycogen synthesis [17]. Furthermore, the phosphorylation of FOXO1 suppresses FOXO1-regulated expression of pyruvate dehydrogenase kinase 4 [18]. Phosphorylated AS160 mediates the translocation of the glucose transporter type 4 (GLUT4) from the cytoplasm to the plasma membrane [19]. This reaction ultimately completes the action of insulin by increasing glucose utilization and storage, as well as promoting glucose uptake in the tissues.
Oxidative stress and insulin resistance
The insulin-mediated signaling pathway plays a crucial role in lowering blood glucose and regulating overall glucose metabolism in vivo. A reduction in the biological effect of insulin is called insulin resistance, in which blood glucose cannot be used effectively as an energy source due to decreased insulin responsiveness of insulin-sensitive tissues such as skeletal muscle, adipose tissue, and the liver. To escape this condition, β-cells produce more insulin, but this eventually leads to β-cell exhaustion [20]. A reduction in insulin secretion due to β-cell exhaustion increases blood glucose levels, resulting in the development of type 2 diabetes [20]. Although defects in insulin receptors contribute to insulin resistance, perturbations in intracellular insulin signaling pathways are mostly responsible for insulin resistance.
Free fatty acids (FFAs) in plasma are generally supplied through lipolysis and are used as a primary energy source through β-oxidation in the liver, heart, and skeletal muscle during fasting and exercise. However, chronically elevated levels of FFAs in the plasma are one of the key triggers leading to defects in the insulin signaling pathway [21,22]. Increased utilization of FFAs leads to the accumulation of fat metabolites, such as fatty acyl-coenzyme A, ceramides, and diacylglycerol in the liver and skeletal muscle [23-25]. High levels of fatty acid metabolites activate serine-threonine kinases, including protein kinase C (PKC), c-Jun N-terminal kinase (JNK), and inhibitory κB kinase (IKK) β, leading to the suppression of insulin-stimulated signaling pathways [23,26,27]. Serine phosphorylation of IRS by these serine-threonine kinases inhibits tyrosine phosphorylation of IRS by insulin, eventually reducing glucose uptake [27].
Recently, fatty acid-induced oxidative stress has drawn attention as one of the causes of insulin resistance. Increased plasma levels of FFAs enhance cellular ROS production [28]. Since mitochondria are a major source of ROS, mitochondrial overload caused by an excess of substrate has been regarded as the main cause of ROS production [29,30]. Recently, the fatty acid metabolite, ceramide, and inflammatory cytokines have also been shown to increase mitochondrial ROS generation [31].
Similar to fatty acid metabolites, ROS also activate PKC, IKK, and JNK, leading to inhibition of IRS tyrosine phosphorylation [28,32] (Fig. 3). Moreover, the activation of nuclear factor-κB by IKK exacerbates insulin resistance by increasing inflammatory cytokines [31]. ROS induce GLUT4 degradation by transporting GLUT4 to the lysosome instead of the plasma membrane via disruption of the retromer complex in a casein kinase 2-dependent manner [33]. In addition, peroxynitrite produced by the accumulation of nitric oxide inhibits the action of insulin by inducing the nitration of tyrosine residues and reducing the phosphorylation of IRS-1 [34]. The activation of AKT is also suppressed by peroxynitrite [35].
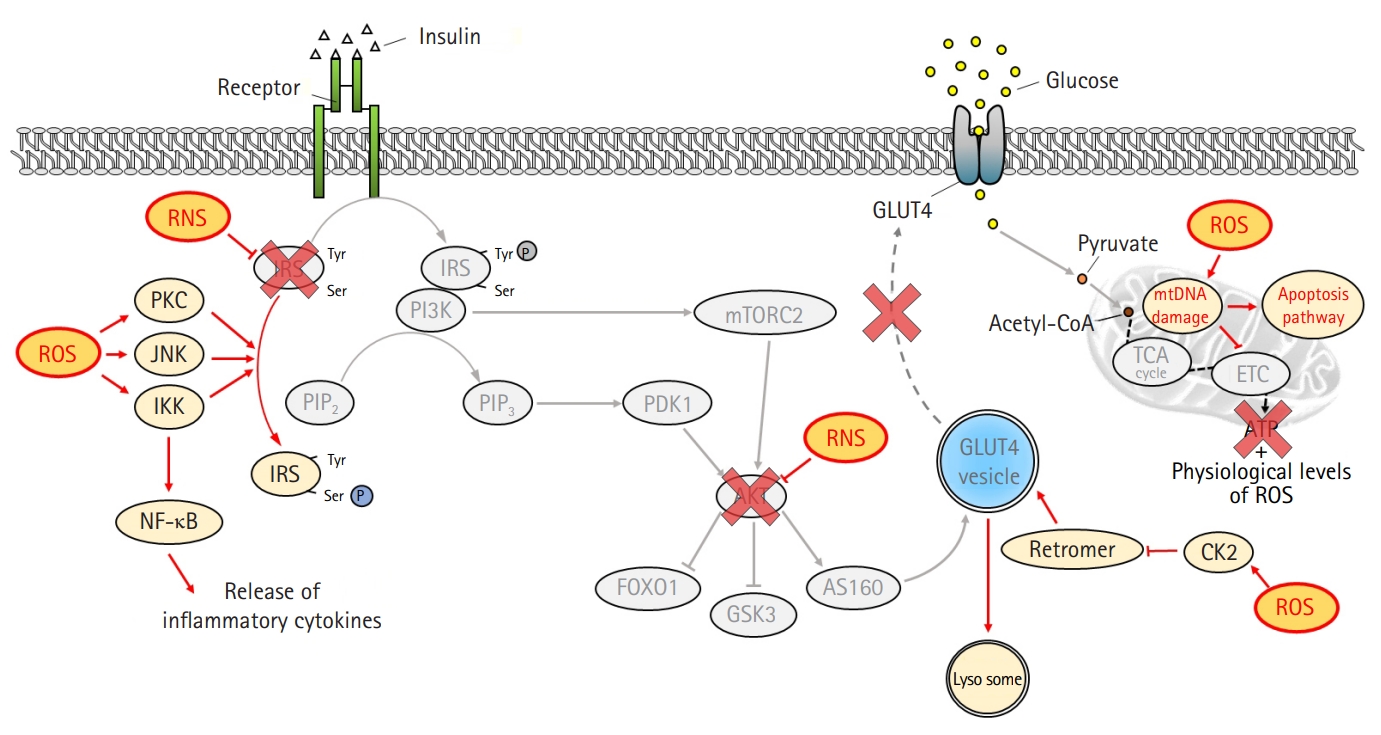
Inhibition of the insulin signaling pathway by oxidative stress. Reactive oxygen species (ROS) interferes with insulin action by altering several substrates of the insulin signaling pathway. ROS activates serine/threonine kinases, including protein kinase C (PKC), c-Jun N-terminal kinase (JNK), and inhibitory κB kinase (IKK), which not only inhibit the activation of insulin receptor substrate (IRS) through serine phosphorylation but also induce inflammation by activating nuclear factor κB (NF-κB). In addition, ROS suppresses glucose absorption by degrading glucose transporter type 4 (GLUT4) in a casein kinase 2 (CK2)-dependent manner. Reactive nitrogen species (RNS) inhibits tyrosine phosphorylation of IRS and protein kinase B (AKT) activation by inducing nitration of tyrosine. Mitochondrial functional defects by ROS not only induce an explosive increase in oxidative stress but also suppress mitochondrial energy production, eventually leading to cell death. Tyr, tyrosine; Ser, serine; PIP2, phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol (3,4,5)-triphosphate; PI3K, phosphatidylinositol 3-kinases; PDK1, phosphatidylinositide-dependent protein kinase 1; mTORC2, mammalian target of rapamycin complex 2; FOXO1, forkhead box protein O1; GSK3, glycogen synthase kinase 3; AS160, AKT substrate of 160 kDa; acetyl-CoA, acetyl coenzyme A; mtDNA, mitochondrial DNA; TCA, tricarboxylic acid cycle; ETC, electron transport chain.
Increased ROS levels suppress the action of insulin by inducing mitochondrial dysfunction [36]. ROS induce mutations in the mitochondrial DNA, resulting in functional defects caused by altered expression of constituent proteins that are important for electron transport, such as complexes I, III, and IV [37]. These functional defects amplify oxidative stress and shut down mitochondrial energy production [38]. Therefore, the vicious cycle formed between ROS and mitochondria eventually leads to apoptosis.
Genetic modification of antioxidant enzymes and insulin sensitivity
Obesity induced by a chronic high-fat diet (HFD) has been shown to increase oxidative stress accompanied by insulin resistance in insulin-sensitive tissues in rodents [39,40]. Generally, the genetic deletion of antioxidant enzymes increases oxidative stress and induces insulin resistance and/or glucose intolerance, while overexpression of antioxidants reduces oxidative stress and improves insulin resistance and/or glucose intolerance (Table 1) [39-64].
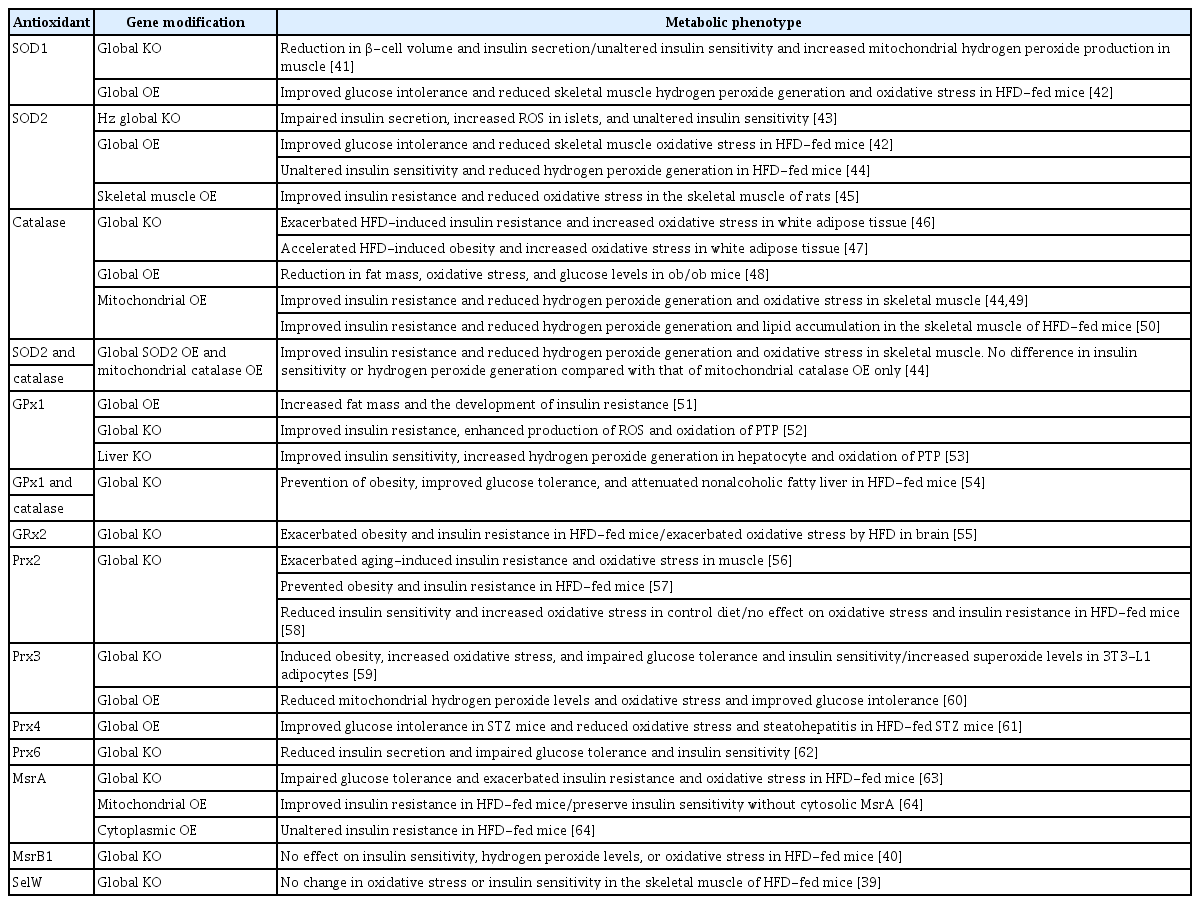
The effect of deficiency or overexpression of antioxidant enzymes on glucose metabolism and insulin sensitivity in mice
However, although ROS levels have been altered by genetic modulation of antioxidant enzymes, some of the studies reported unaltered insulin sensitivity, especially in the case of SOD modulation alone (Table 1). Moreover, unexpectedly, the global knockout of GPx1 improves insulin resistance [52], whereas overexpression of GPx1 impairs insulin sensitivity [51]. Although increased ROS levels have been known to suppress insulin signaling pathways through activation of serine/threonine kinases [65,66], paradoxical activation of insulin signaling pathways by ROS through oxidation of protein-tyrosine phosphatase 1B has been reported in GPx1-knockout mice [52]. Therefore, increased ROS may be associated with a combination of both favorable and unfavorable effects on insulin sensitivity.
In addition to the results of genetically modified mouse models, the treatment of experimental animals with antioxidants, such as hemin, glutathione, vitamin C, and polyphenols, has been reported to improve insulin resistance [67-70], suggesting that a reduction in oxidative stress could be a potential therapeutic approach for type 2 diabetes.
Oxidative stress in patients with type 2 diabetes
There is a large body of evidence indicating that oxidative stress is increased in the plasma or blood cells of diabetic patients. Compared with those in healthy glucose-tolerant individuals, the levels of biomarkers of oxidative damage to proteins [71,72], lipids [73-76], and DNA [74,77], such as carbonyl groups, malondialdehyde, thiobarbituric acid reactive substances, and 8-hydroxydeoxyguanosine, are increased in patients with type 2 diabetes. Moreover, these biomarker levels are positively correlated with hemoglobin A1c (HbA1c) or homeostatic model assessment for insulin resistance (HOMA-IR) in patients with type 2 diabetes [76-79], suggesting a close link between insulin resistance and oxidative stress.
Oxidative stress is higher in patients with diabetes than in healthy individuals, not only in the plasma but also in insulin-sensitive peripheral tissues, including skeletal muscles. Higher levels of biomarkers of DNA oxidation [80] and lipid oxidation [81] have been reported in skeletal muscle tissues of diabetic patients as compared to healthy controls. Lipid peroxidation levels are negatively correlated with glucose disposal [81]. Moreover, in a previous study assessing nitrosative stress in diabetes, nitrites and nitrates were increased in quadriceps muscle of diabetic patients as compared to the control group, and nitrotyrosine levels positively correlated with HbA1c levels [82]. However, even though the levels of oxidative stress markers are higher in patients with type 2 diabetes, some studies do not support the positive correlation between HbA1c and oxidative stress markers [83,84].
Considering the critical role of oxidative stress in the development of type 2 diabetes, the levels of oxidative stress markers are thought to increase in the prediabetic state. Consistent with this hypothesis, the plasma levels of thiobarbituric acid reactive substances have been shown to positively correlate with body mass index and waist circumference in obese, nondiabetic individuals [85]. Moreover, higher levels of mitochondrial ROS have been detected in the skeletal muscle tissue of obese human subjects as compared with lean controls [86,87]. Higher levels of circulating FFAs might be implicated in increased ROS levels in obese subjects, as suggested by previous studies. Two-consecutive fat-rich meals have been shown to increase plasma malondialdehyde levels in healthy young males [88]. Additionally, in nonobese sedentary humans, overfeeding with an HFD for 28 days induced insulin resistance and increased muscle protein carbonylation [89]. Furthermore, increased levels of carbonylated protein negatively correlated with insulin sensitivity in these overfed subjects [89].
As excess ROS are scavenged by the antioxidant system, increased oxidative stress in obese and type 2 diabetic subjects is followed by a reduction in antioxidant levels. Obese patients with body mass indexes above 35 kg/m2 exhibit low levels of carotenoids and vitamin E [90]. Lower levels of vitamin E have been reported in plasma of patients with type 2 diabetes as compared to normal controls [91]. In the Rotterdam Study, the ferric reducing ability of plasma (FRAP) score, an index of dietary antioxidant capacity, was inversely related to the insulin resistance index [92]. Moreover, increased levels of total dietary antioxidant capacity were associated with a reduced risk of type 2 diabetes in a female cohort study [93]. However, not all studies support the close relationship between antioxidant levels and the risk of type 2 diabetes. No association has been found between the serum levels of α-tocopherol or β-carotene and the risk of diabetes in middle-aged males [94]. Moreover, in a cohort study, levels of dietary antioxidants, including vitamin C, vitamin E, carotenoids, flavonoids and flavones, were not associated with the risk of type 2 diabetes in middle-aged male smokers [55].
Additionally, the administration of antioxidants to humans has shown controversial results. Increased dietary intake of β-carotene for 10 years have been associated with reduced risk of diabetes [95]. Vitamin C intake is associated with a reduced risk of incident diabetes in Japanese women [96]. In contrast, the administration of α-tocopherol or β-carotene does not significantly affect the occurrence of diabetes in males [94]. Moreover, resveratrol-mediated attenuation of ROS production and oxidative damage does not affect HOMA-IR in patients with type 2 diabetes [97].
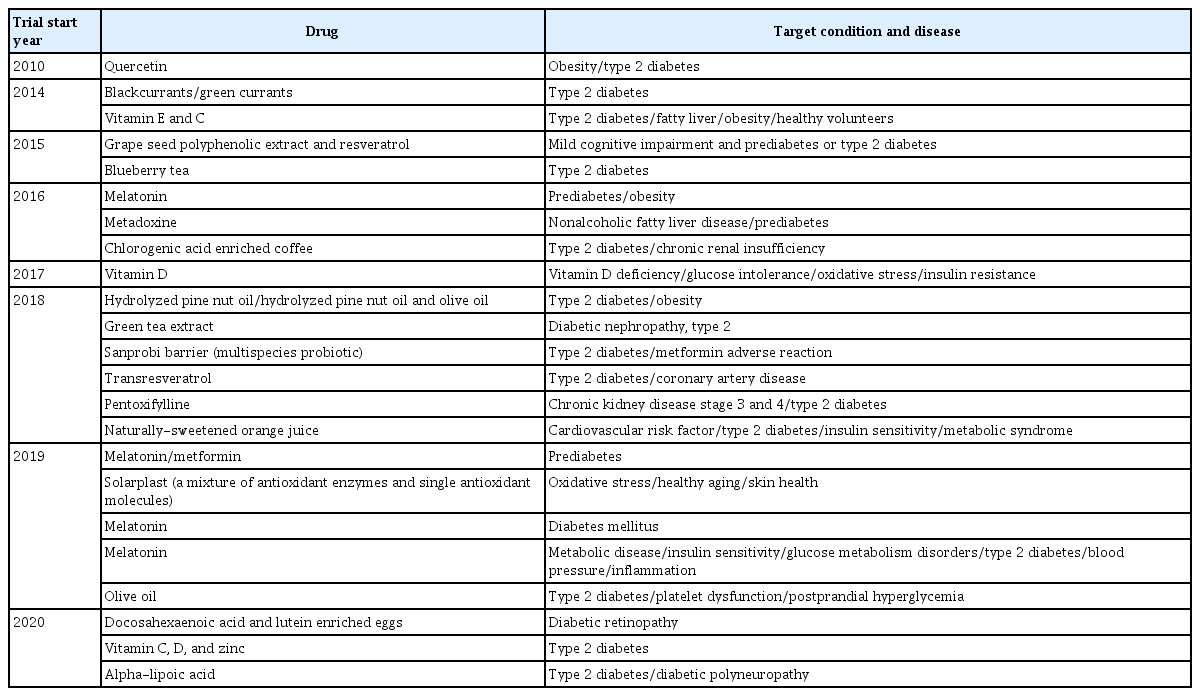
Based on experimental animal and human studies, oxidative stress is closely linked to type 2 diabetes. However, the antioxidant capacity of the serum or tissue is not consistently related to the risk of type 2 diabetes. Additionally, the administration of antioxidants to prediabetic and diabetic patients has produced inconsistent results. There might be several reasons for this inconsistency. A limited number of antioxidants have been assessed for antioxidant capacity in serum. Additionally, it is difficult to assess the antioxidant capacity in insulin-sensitive tissues such as skeletal muscle and adipose tissue in humans. Serum levels of antioxidants are not always consistent with tissue levels. Furthermore, some of the currently used drugs for the treatment of type 2 diabetes have been shown to exert an antioxidant property which may conceal the therapeutic effect of vitamins and natural antioxidants in type 2 diabetes [98]. In most prospective studies, limited types of antioxidants have been used to assess the risk of type 2 diabetes. However, antioxidants still hold promise as potential therapeutic options for the prevention and treatment of type 2 diabetes and its complications. According to Clinicaltrials.gov, 23 clinical trials are recruiting or planning to recruit participants to take part in studies examining the effects of antioxidants on glucose levels or peripheral tissue complications in prediabetic and type 2 diabetic patients (Table 2). Dietary and natural antioxidants from various sources and vitamins are still popular agents for clinical trials, and melatonin is emerging as a potential therapy for type 2 diabetes. Pharmaceuticals targeting prooxidant and antioxidant enzymes, such as NADPH oxidase inhibitors, xanthine oxidase inhibitors, and SOD mimetics, are actively being developed for the treatment of oxidative stress-related diseases, including type 2 diabetes [99,100].
Conclusion
Oxidative stress is elevated in obese prediabetic and type 2 diabetic experimental animals and humans. However, although experimental animal studies show promising results, the effects of antioxidant administration on type 2 diabetes in humans are still inconsistent. Nevertheless, clinical trials examining the therapeutic effects of dietary antioxidants and vitamins on type 2 diabetes and its complications are still ongoing. Pharmaceuticals targeting redox regulating enzymes are actively under development, and the successful development of pharmaceuticals might help us understand the therapeutic effectiveness of antioxidants in the treatment of type 2 diabetes.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This research was supported by grants from the Medical Research Center Program (2015R1A5A2009124) and the Basic Science Research Program (2019R1A2C1088730) through the National Research Foundation of Korea (NRF), funded by the Korean government.
Author contributions
Conceptualization, Funding acquisition, Validation, Formal analysis, Supervision: SYP; Writing-original draft: SP, SYP; Writing-review & editing: SP, SYP.