Frailty and elderly in urology: implications for postoperative complications
Article information
Abstract
The geriatric population is at a greater risk of postoperative complications than young adults. This risk is associated with the physiologic decline seen in this population known as frailty. Unlike fitter patients, frail patients who undergo operative treatment have a greater likelihood of developing postoperative complications and endure prolonged hospital stays. This circumstance is comparable to the urological status. Therefore, tolerable measurement of frailty as a domain of preoperative health status has been suggested to ascertain vulnerability in elderly patients. In this review, we will elaborate on the concept of frailty and examine its importance with respect to surgical complications, focusing on the urological status.
Introduction
The demographic composition of the surgical population changes with the aging of the population. Older adults account for an increasing proportion of the surgical population, with >35% of all inpatient operations being performed in adults aged 65 years or older in the United States. This proportion is higher in medicine subspecialties, such as urology, where 65% of all surgeries are performed in adults aged 65 years and older [1] and is anticipated to increase in the future. This situation is similar in every country including South Korea. Therefore, it is essential to understand the unique physiology and characteristics of older adults to provide optimal urologic care for these patients.
The geriatric population is at a greater risk for postoperative complications than young adults. This risk is associated with the physiologic decline observed in this population known as frailty. Frailty is a state of decreased physiologic reserve that increases a patient’s susceptibility to incapacity. Thus, by definition, frailty increases the risk of poor surgical outcomes. A few studies have reported outcomes, such as a higher risk of delirium, injury, intensive care unit (ICU) admissions, ICU stay, and death, in geriatric urologic patients than in young adults [2,3].
Previous concepts of postoperative risk estimation, such as the American Society of Anesthesiology (ASA) physical status classification and European Cooperative Oncology Group (ECOG) performance, have focused on single-organ systems to determine the risk of adverse postoperative cardiac, hepatic, pulmonary, or renal events [4,5]. Although these algorithms continue to play a role in the postoperative risk estimation of urologic patients, frailty has covered these strategies as an effective, efficient, and global estimation for surgical risk and represents a notable paradigm shift for the anticipation of postoperative complications [6]. In this review, we will elaborate on the concept of frailty and examine its importance with respect to surgical complications, with a focus on urological status.
Definition of frailty
Although no single operational definition is all-encompassing, a clear conceptual framework for frailty has been established. Frailty is a state of extreme vulnerability to stressors that induces adverse health outcomes [7,8]. However, frailty is a complex, multidimensional, and cyclical state of decreased physiologic reserve that results in diminished resilience and adaptive capacity and increased vulnerability to stressors (Fig. 1) [9]. Frailty has also been related to the concept of health deficits, that, when accumulated over time, heightens an individual’s vulnerability to adverse health outcomes [10].

Model for defining frailty. Fit patients have robust adaptive capacity and resilience to stressors, which lead to more favorable outcomes. Pre-frail patients have weakened adaptive capacity and resilience to stressors, and frail patients have poor adaptive capacity and resilience to stressors. Adapted from Ethun et al. [9] with permission of Wiley.
The prevalence of frailty is high among the elderly and increases with age, as observed in 40% of patients aged 80 years or older compared with 10% of patients aged between 65 and 75 years [11]. Unlike fitter patients, frail patients who undergo surgery have a greater likelihood of developing postoperative complications, being discharged to care facilities, and having longer hospital stays. Postoperative complications can result in a series of events leading to loss of independence, decline in the quality of life, disability, increased healthcare costs, and even death [2,3]. Therefore, adequate measurement of frailty as a domain of preoperative health status has been proposed to ascertain vulnerability in elderly patients.
Measurement of frailty
Current recommendations state that all patients aged >70 years and those with significant weight loss (>5%) due to chronic illness should be screened for frailty [9]. However, it is not clear which frailty measure is optimal for screening and assessment. Over 70 different tools exist to measure frailty, few of which have been proven, and they range from a single item to more than 90 items. They also range in their intended purpose, with some frailty systems being designed as screening tools to risk-stratify patients, and others as more formal frailty estimations aimed at guiding treatment strategy. A brief summary of the most commonly used frailty assessment tools is provided below.
1. Individual assessment tools
Using a single-item estimation tool is a quick and easy means to quantify a patient’s level of frailty. The most commonly used single-item tools that have been demonstrated to be reliable predictors of frailty are gait speed (the measured time it takes for a patient to walk a 5-m distance), grip strength (a marker of frailty via universal loss of muscle mass or myopathy associated with decreased physiologic reserve), and Timed Up-and-Go score (the measured time it takes for a patient to rise from a chair, walk 10 feet, turn around, and return to being seated) [12-14].
Although these single-item assessments can be convenient to use in a busy and time-constrained circumstance, they can also lack sensitivity and specificity and, when in isolation, should be used with caution [6].
2. FRAIL scale and Vulnerability Elders Survey-13
Developed by the Geriatric Advisory Panel of the International Academy of Nutrition and Aging, the fatigue, resistance, ambulation, illness, and loss of weight (FRAIL) scale is a validated screening method consisting of five straightforward questions (Table 1) [9,15]. Because it can be self-administered and does not crave a face-to-face examination, this tool can be an efficient and cost-effective tool to screen large groups of patients for frailty. However, this scale is applied most frequently in primary care or community environments and has not been investigated extensively as a screening method for patients with cancer [9,16].
The Vulnerability Elders Survey-13 (VES-13) is also a self-administered survey consisting of 13 items: one item for age and 12 for self-rated health, physical ability, and functional performance. However, unlike the FRAIL scale, this practical screening tool can be used as a reliable marker of frailty in patients with cancer. Despite these advantages, the VES-13 may be inaccurate because of patients’ overestimation of their own competencies [17,18].
3. Phenotypic frailty
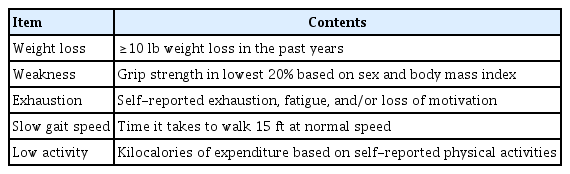
Phenotypic frailty is one of the most widely used frailty assessment tools in oncology and has been identified by the American Geriatric Society and the American College of Surgeons (ACS) as an adequate strategy for preoperatively measuring elderly patients. It is based on the notion that frailty results from age-associated biological changes across multiple domains, such as nutrition and energy metabolism. This method consists of five items (weight, strength, energy, speed, and activity) and needs a combination of questionnaires and in-office estimations (Table 2) [9,19].
4. Frailty index and modified frailty index
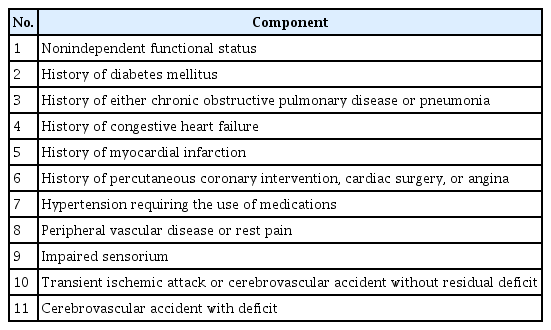
The frailty index (FI) was initiated from the Canadian Study of Health and Aging and is based on an accumulative deficit model [20]. This method proposes that the accumulation of medical, functional, and social shortfalls over an individual’s lifetime induces a nonspecific, age-associated vulnerability, or, in other words, frailty [8]. The original FI includes 70 items, which vary from vague to specific symptoms, signs, diseases, and disabilities. Although many of the included items can be found in patient charts, several need more cumbersome and labor-intensive estimations, which makes FI less attractive in clinical practice. Therefore, Obeid et al. [21] proposed a modified FI (mFI), which maps the 70 variables from the original FI into 11 preexisting variables from the National Surgical Quality Improvement Program (NSQIP) database and has since been backed by the ACS (Table 3) [9].
5. Comprehensive geriatric assessment
Comprehensive geriatric assessment (CGA), a multidimensional measurement process for identifying and managing elderly patients, is one of the most extensively investigated and used methods in oncology. Using principles similar to those of the cumulative deficit model, the CGA focuses on some domains of a patient’s psychosocial, medical, and functional abilities and can be a reliable assessment of frailty when used as a screening method in patients with cancer [22]. However, with 64 instruments of assessment, managing a full CGA can take hours to complete and is often impractical; hence, the CGA was altered to address these issues. For example, the Cancer-Specific Geriatric Assessment is a brief and more focused method that combines both self-administered and in-office assisted estimations [23]. It contains six of the nine domains from the full CGA, and the methods for measuring those domains were specifically chosen for their reliability, brevity, validity, and prognostic ability in patients with cancer [24].
Frailty and postoperative complications, especially with respect to urological surgery
The decision regarding a patient’s “fitness” for operative treatment has traditionally been based on fairly subjective and particularly simplistic assessments, which can be limited in their capacity to predict postoperative morbidity and mortality [13]. Because it transcends age or any single-organ system, frailty has been shown to be a stronger predictor of postoperative complications than some previous surgical risk-assessment methods [25,26]. Revenig et al. [27] reported that frailty was even predictive of postoperative complications among patients undergoing minimally invasive abdominal surgery.
Although less well investigated, the value of frailty, especially in urological oncologic surgery, is increasingly being investigated. It has been demonstrated that frailty is associated with worse long-term and short-term survival in patients undergoing surgery for various malignancies. For example, when frail patients were assessed using the NSQIP mFI, they demonstrated higher 30-day mortality rates than nonfrail patients undergoing surgery for bladder cancer (3.5% vs. 1.8%; p=0.01) [28]. Expanding the mFI to include 15 variables, Lascano et al. [29] found that, in patients undergoing operative treatments for urologic malignancies, such as cystectomy, prostatectomy, nephrectomy, and nephroureterectomy, there was a two to six times increased risk of death within 30 days for every 0.05 increase in the calculated mFI compared with that in nonfrail patients (mFI <0.05). They also reported that patients undergoing operative treatments for urologic malignancies with high frailty (mFI >0.20) had a significantly increased risk of major side effects (Clavien-Dindo grade IV) compared with nonfrail patients (odds ratio, 3.70; 95% confidence interval, 2.87–7.79; p<0.0005).
The mFI has also been used to evaluate patients treated with robotic-assisted radical prostatectomy (RARP) for prostate cancer. Levy et al. [30] also queried the NSQIP database to create a dataset of 23,000 patients who underwent RARP. An mFI score of ≥3 was related to a 12-fold increased risk of a Clavien-Dindo grade IV event compared with that in nonfrail patients.
Conclusion
Current knowledge on preoperative geriatric estimation in urologic patients is sparse. Frailty is emerging as one of the most significant predictors of postoperative complications, disease progression, and death. Therefore, preoperative recognition of frailty in such patients seems to be an important method in urological practice. Moreover, adequate stratification of preoperative frailty may induce a decrease in postoperative complications. It is also important that high-risk patients are routinely instructed to undergo training such as physical therapy, walking, and use of incentive spirometry in an effort to reduce postoperative complications. Thus, further research in urological environments, especially in multicenter randomized controlled trials, is required to develop a standardized cutoff value for frailty to provide better urologic patient care.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.