Primary hepatic sarcoidosis presenting with cholestatic liver disease and mimicking primary biliary cholangitis: a case report
Article information
Abstract
Sarcoidosis often involves the liver. However, primary hepatic sarcoidosis confined to the liver without evidence of systemic involvement is rare. We report the case of a 37-year-old man with hepatic sarcoidosis who initially presented with elevated liver enzymes and suspicious cirrhotic nodules on computed tomography. The patient had cirrhosis but did not have portal hypertension. Based on the initial histopathologic finding of chronic granulomatous inflammation and the common clinical characteristics of sarcoidosis, he was initially diagnosed with primary biliary cholangitis, and his daily dosage of ursodeoxycholic acid was increased to 900 mg. After 14 months of treatment, his total serum bilirubin concentration was 10.9 mg/dL (upper normal limit, 1.2 mg/dL). Additionally, a transjugular liver biopsy revealed multiple noncaseating granulomas. He was diagnosed with primary hepatic sarcoidosis involving the lungs, heart, spleen, kidneys, and skin. Treatment with methylprednisolone was initiated. Two weeks later, he was started on azathioprine, and the dose of steroid was simultaneously reduced. These findings indicate the importance of including hepatic sarcoidosis as a possible diagnosis in patients with elevated liver enzymes or cryptogenic cirrhosis.
Introduction
Sarcoidosis is a multisystem disease characterized by the formation of noncaseating granulomas in affected organs. Sarcoidosis can affect almost all organs, with more than 90% of patients showing involvement of the lungs and hilar lymph nodes. The liver is the third most commonly affected organ [1]. Most patients with hepatic involvement are asymptomatic, but 5% to 30% of these patients present with abdominal pain in the right upper quadrant, nausea, vomiting, and/or hepatomegaly [2-4]. A small number of patients with hepatic sarcoidosis present with chronic cholestasis, cirrhosis, portal hypertension, and Budd-Chiari syndrome [5,6]. Cirrhosis occurs in less than 1% of patients with sarcoidosis [5]. This study reports the case of a patient who presented with elevated liver enzymes and abnormal hepatic morphology as the first clinical signs of systemic sarcoidosis and was ultimately diagnosed with systemic sarcoidosis.
Case
A 37-year-old man visited our outpatient clinic with elevated liver enzymes and computed tomography (CT) findings indicative of liver cirrhosis. The patient reported that his liver enzymes had been elevated during the previous 4 to 5 years, but he was not further tested. He had not been diagnosed with any disease and had not taken any medications or had traveled. At presentation, the patient was asymptomatic; his liver and spleen were not palpable, and there were no signs of chronic liver disease, such as palmar erythema, spider nevi, gynecomastia, and ascites, and no unusual skin lesions. The patient was 187 cm tall and weighed 104 kg (body mass index, 29.7 kg/m2), drank alcohol 2 to 3 times per month, and was a current smoker with a history of seven pack-years. His maternal grandfather had hepatocellular carcinoma.
Laboratory examination showed elevated concentrations of liver enzymes, including aspartate aminotransferase (AST, 133 IU/L; upper normal limit [UNL], 40 IU/L), alanine aminotransferase (ALT, 158 IU/L; UNL, 40 IU/L), alkaline phosphatase (ALP, 378 IU/L; UNL, 129 IU/L), and gamma-glutamyl transferase (GGT, 2,162 IU/L; UNL, 73 IU/L). However, serum bilirubin (0.71 mg/dL) and albumin (5.0 g/dL) concentrations were not elevated and the prothrombin time was not prolonged (11.8 seconds). Abdominal CT showed cirrhotic nodules in both lobes of the liver (Fig. 1A), but a chest X-ray showed no evidence of hilar lymphadenopathy (Fig. 1B).
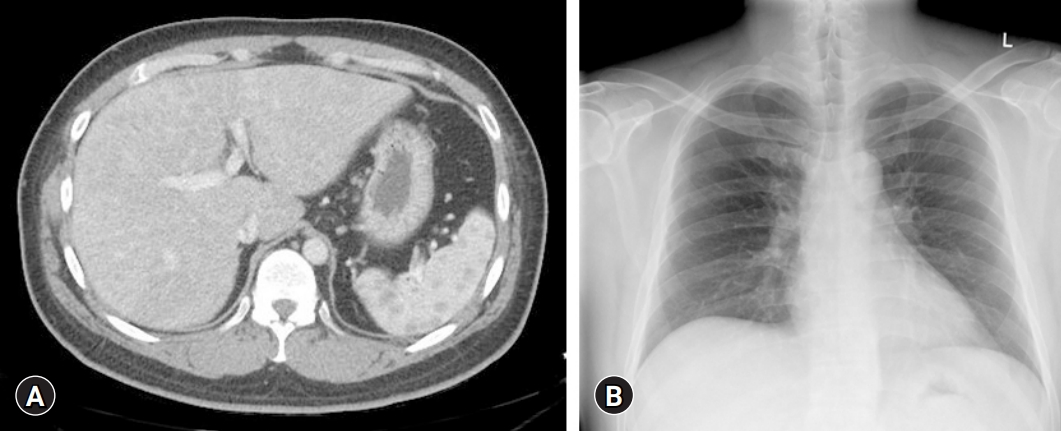
Initial abdominal computed tomography (CT) and chest X-ray of the patient. (A) Enhanced CT image of the abdomen in the portal-venous phase, showing underlying liver cirrhosis with multiple cirrhotic nodules in both lobes of the liver. (B) The first chest X-ray shows no evidence of hilar lymphadenopathy.
The elevated liver enzymes and abdominal CT findings, showing multiple cirrhotic nodules in both lobes, led to further laboratory evaluations to determine the cause of liver cirrhosis. Serological tests for hepatitis A, B, and C viruses were all negative, and levels of antimitochondrial antibody (AMA), anti-smooth muscle antibody, anti-liver kidney microsome type 1, antineutrophil cytoplasmic antibody, alpha-1-antitrypsin, ceruloplasmin, fluorescent antinuclear antibody, and quantitative copper were all within normal ranges. Serum concentrations of immunoglobulins (Igs), including IgG (1,440 mg/dL), IgA (289 mg/dL), and IgM (120 mg/dL), were not elevated.
Because treatment with the usual dose of ursodeoxycholic acid (UDCA, 600 mg/day) for 5 months had no effect on liver enzyme levels and all serologic markers were negative, a transjugular liver biopsy was performed. Histopathologic evaluation of the specimen indicated chronic granulomatous inflammation (Fig. 2), with no staining for fungi or acid-fast bacilli, and a polymerase chain reaction test negative for Mycobacterium tuberculosis. Based on the biopsy results and the clinical characteristics of sarcoidosis, he was initially diagnosed with primary biliary cholangitis (PBC), and his daily dosage of UDCA was increased to 900 mg. After 6 months of treatment, however, his liver enzyme levels did not decrease significantly, and a follow-up chest X-ray showed no abnormal findings.
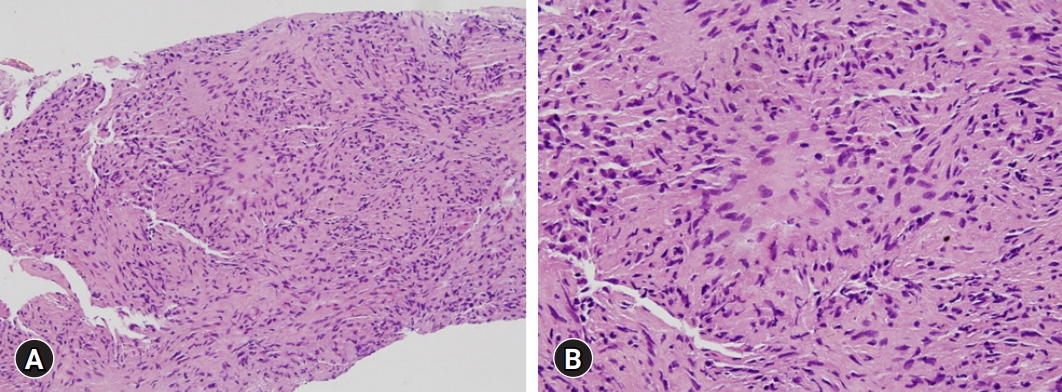
Histopathology of the first transjugular liver biopsy. (A) The liver shows chronic granulomatous inflammation without necrosis. (B) Focal aggregates of epithelioid histiocytes are present (hematoxylin and eosin stain; [A] ×200, [B] x400).
Routine laboratory testing after the patient had taken 900 mg/day UDCA for 14 months showed a total bilirubin concentration of 10.9 mg/dL (UNL, 1.2 mg/dL), and the patient was hospitalized. Abdominal CT showed innumerable and scattered low-attenuation lesions in the liver parenchyma, spleen, and kidneys, as well as multiple small lymph nodes at the lower paraesophageal, perigastric, portocaval, retrocaval, paraaortic, retroperitoneal, liver hilum, hepatoduodenal ligament, common hepatic artery, and celiac axis areas. After admission, erythematous plaques were observed on the right elbow and left knee (Fig. 3A). Biopsy specimens from these areas indicated the presence of epithelioid cell granulomas (Fig. 3B). Based on the results of the skin biopsy, he was diagnosed with sarcoidosis, a diagnosis confirmed by his serum angiotensin-converting enzyme (ACE) levels elevated to 92.2 U/L (UNL, 47 U/L) and the results of chest and abdominal CT. The patient underwent transthoracic echocardiography and fundus examination to identify the extent of sarcoidosis. Examination of a second transjugular liver biopsy sample showed multiple confluent noncaseating granulomas in the hepatic capsule, portal and focal periportal inflammation, mild lobular activity, and portal fibrosis (Fig. 4). These results confirmed the diagnosis of hepatic sarcoidosis, accompanied by involvement of the lungs, heart, spleen, kidneys, and skin (Supplementary Fig. 1).
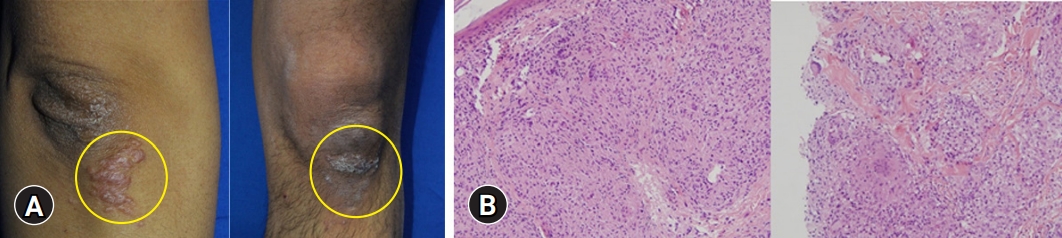
Clinical and histologic findings of the skin. (A) Erythematous plaques are present on the right elbow and left knee. (B) The skin shows noncaseating granulomas composed of epithelioid histiocytes (hematoxylin and eosin stain, ×200).
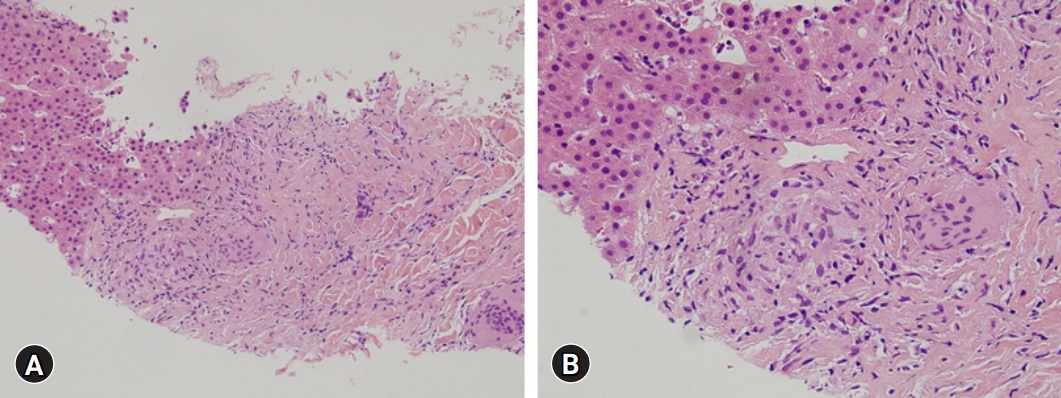
Histopathology of the second transjugular liver biopsy. (A, B) The liver shows multiple noncaseating granulomas in the hepatic capsule and portal tracts (hematoxylin and eosin stain; A, ×200; B, ×400).
After confirmation of sarcoidosis, the patient was administered intravenous methylprednisolone (40 mg/day for 3 days) followed by oral prednisolone (80 mg/day for 3 days and then tapered down to 40 mg/day). The patient was started on azathioprine (25 mg/day) 2 weeks after initiation of prednisolone, and the dose of steroid was simultaneously reduced by 5 mg/day every 3 days. After 3 weeks of steroid treatment, his liver enzyme concentrations had markedly decreased (AST, 95 IU/L; ALT, 62 IU/L; ALP, 379 IU/L; total bilirubin, 2.32 mg/dL) and the patient was discharged from the hospital. At follow-up 49 months after the initiation of steroid treatment, the patient was taking prednisolone (10 mg/day) and azathioprine (100 mg/day), and blood chemistry indicated continued improvements in liver enzymes (AST, 46 IU/L; ALT, 45 IU/L; ALP, 174 IU/L; total bilirubin, 0.98 mg/dL; GGT, 790 IU/L) (Supplementary Fig. 2).
Discussion
The present report describes the procedures used to diagnose sarcoidosis involving the liver, lungs, heart, spleen, kidneys, and skin in a patient who initially presented with elevated liver enzymes and abnormal hepatic morphology. The characteristics of this patient were unusual in that an elevation in liver enzymes was the first and only clinical finding of systemic sarcoidosis, and there was no initial involvement of the lungs or skin. Although hepatic involvement has been observed in 5% to 30% of patients with sarcoidosis [3,7], isolated liver involvement without lung disease is rare and observed in only 13% of patients with sarcoidosis [7].
On initial examination, abdominal CT of the patient revealed cirrhotic hepatic nodules, whereas laboratory tests showed elevated liver enzymes, findings suggestive of cirrhosis. A liver biopsy, however, showed mild hepatocellular cholestasis and portal fibrosis, providing no pathological evidence of cirrhosis. In addition, the CT scan provided no evidence of portal hypertension, such as dilatation of the portal and/or mesenteric vein, contrast enhancement of the paraumbilical vein, collateral vessels or varices, splenomegaly, or ascites. Sarcoidosis and granulomatous liver disease have been classified into four broad categories [8], with the present patient having group III sarcoidosis, which is characterized by clinical and laboratory evidence of hepatocellular disease with or without portal hypertension. The prevalence of liver cirrhosis at the time of diagnosis of hepatic sarcoidosis has been reported to range from 6% to 24% [3,7,9-11], with signs of liver cirrhosis and portal hypertension observed in up to 18% of patients with hepatic sarcoidosis [3,4,12].
Although the initial liver biopsy confirmed hepatic granulomas, the final diagnosis of sarcoidosis was delayed. This patient was negative for AMA, had normal IgM levels, and bile duct inflammation was unclear in the liver biopsy sample. These findings suggested an initial diagnosis of PBC, because less than 10% of patients with sarcoidosis have isolated extrapulmonary sarcoidosis [13], and because cirrhosis is not common in patients with hepatic sarcoidosis [5]. Although noncaseating granulomas are characteristic of sarcoidosis and occur in 60% to 80% of patients [2], the presence of hepatic granulomas on a diagnostic liver biopsy is also consistent with a broad spectrum of other diseases, including PBC, infectious diseases, drug reactions, and cancer [14].
Hepatic sarcoidosis can manifest as intra- or extrahepatic cholestasis [15]. Intrahepatic cholestasis in patients with hepatic sarcoidosis is characterized histologically by numerous well-formed granulomas located mainly in the periportal area. In comparison, PBC manifests as poorly demarcated granulomas located in portal tracts along damaged bile ducts [17], whereas primary sclerosing cholangitis is characterized by prominent concentric periductal fibrosis [18]. Extrahepatic cholestasis in patients with hepatic sarcoidosis has been associated with the occurrence of extrahepatic obstruction due to narrowing of the common hepatic duct by granulomas located in the biliary tract [15].
During the differential diagnosis of an unexplained increase in liver enzymes, it is important to consider sarcoidosis as a possible cause. Because of the relatively asymptomatic nature of hepatic sarcoidosis and liver enzyme elevation, imaging methods have a decisive role in the diagnosis of hepatic sarcoidosis [9]. The most common liver enzyme abnormalities in sarcoidosis are elevations in ALP and GGT to more than three times their UNLs. AST and ALT elevations are less common, usually less than twice the UNLs, and less severe than ALP and GGT elevations [6,9]. Hyperbilirubinemia is uncommon in patients with sarcoidosis, with all patients presenting with preserved liver synthetic function [9]. The epithelioid cells of sarcoid granulomas may be responsible for the elevation of serum ACE [18], but elevated serum ACE has a sensitivity of only 57% and a specificity of 90% for the diagnosis of sarcoidosis [19].
Guidelines to date have not specified the initial treatment (drug of choice, dose, and duration) of patients with hepatic sarcoidosis [17]. Glucocorticoids are generally administered as first-line therapy [9], but large controlled studies performed to date do not provide supporting evidence for the efficacy and long-term benefits of glucocorticoids and other immunosuppressive agents [15,20]. Although steroid treatment can reduce clinical symptoms, liver enzyme levels, and hepatomegaly [3], it does not prevent the progression of hepatic sarcoidosis [2].
The diagnosis of sarcoidosis in our patient was difficult because the initial clinical findings included only elevated liver enzymes, abnormal hepatic morphology, and no evidence of systemic involvement. To our knowledge, this report describes the first patient to date diagnosed with hepatic sarcoidosis who had elevated hepatic enzyme levels and suspicious cirrhotic nodules on CT and had cirrhosis without portal hypertension. These findings indicate the importance of considering hepatic sarcoidosis as a possible diagnosis in patients with unexplained elevations in liver enzymes or cryptogenic cirrhosis.
Supplementary materials
Supplementary Figs. 1 and 2 can be found via https://doi.org/10.12701/yujm.2021.01151.
Comparison of abdominal computed tomography (CT) findings at the first visit and 24 months later. (A) Initial abdominal CT showing multiple cirrhotic nodules in both lobes. (B) Abdominal CT 24 months later, showing innumerable and scattered low-attenuation lesions in the liver parenchyma, spleen, and both kidneys, and multiple small lymph nodes at the lower paraesophageal, perigastric, portocaval, retrocaval, paraaortic, retroperitoneal, liver hilum, hepatoduodenal ligament, common hepatic artery, and celiac axis areas. (C) Chest CT 24 months later, showing innumerable small nodules with perilymphatic distribution in the right upper and left lower lung.
Clinical course of the patient.
Notes
Ethical statements
This retrospective study was approved by the Institutional Review Board (IRB) of Pusan National University Hospital (IRB No: 2106-033-104), and the requirement for informed consent from the patient was waived by the IRB.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
This research was supported by a clinical research grant from Pusan National University Hospital in 2019.
Author contributions
Conceptualization: JH; Funding acquisition: JH; Data curation: MBK, JA; Writing-original draft: YJP, HYW; Writing-review & editing: YJP, HYW, JH.
