Clinical implication of adjuvant chemotherapy according to mismatch repair status in patients with intermediate-risk stage II colon cancer: a retrospective study
Article information
Abstract
Background
The present study evaluated the clinical implications of adjuvant chemotherapy according to the mismatch repair (MMR) status and clinicopathologic features of patients with intermediate- and high-risk stage II colon cancer (CC).
Methods
This study retrospectively reviewed 5,774 patients who were diagnosed with CC and underwent curative surgical resection at Kyungpook National University Chilgok Hospital. The patients were enrolled according to the following criteria: (1) pathologically diagnosed with primary CC; (2) stage II CC classified based on the 7th edition of the American Joint Committee on Cancer staging system; (3) intermediate- and high-risk features; and (4) available test results for MMR status. A total of 286 patients met these criteria and were included in the study.
Results
Among the 286 patients, 54 (18.9%) were identified as microsatellite instability-high (MSI-H) or deficient MMR (dMMR). Although all the patients identified as MSI-H/dMMR showed better survival outcomes, T4 tumors and adjuvant chemotherapy were identified as independent prognostic factors for survival. For the intermediate-risk patients identified as MSI-low (MSI-L)/microsatellite stable (MSS) or proficient MMR (pMMR), adjuvant chemotherapy exhibited a significantly better disease-free survival (DFS) but had no impact on overall survival (OS). Oxaliplatin-containing regimens showed no association with DFS or OS. Adjuvant chemotherapy was not associated with DFS in intermediate-risk patients identified as MSI-H/dMMR.
Conclusion
The current study found that the use of adjuvant chemotherapy was correlated with better DFS in MSI-L/MSS or pMMR intermediate-risk stage II CC patients.
Introduction
Complete surgical resection followed by adjuvant chemotherapy according to pathologic stage is the current standard of care for patients with locoregional colon cancer (CC). For patients with stage III disease, the standard adjuvant chemotherapy is usually FOLFOX (infusional 5-fluorouracil [5-FU], leucovorin, and oxaliplatin) or CAPOX (capecitabine and oxaliplatin) [1,2]. However, for patients with stage II disease, the additional survival benefit from adjuvant chemotherapy varies according to clinicopathological parameters, including microsatellite instability (MSI). Thus, standard guidelines do not recommend adjuvant therapy for patients with low-risk stage II disease, while recommending adjuvant chemotherapy for patients with high-risk stage II disease (T3N0 with high-risk factor for recurrence or T4N0). High-risk factors include poorly differentiated histology, lymphovascular invasion, perineural invasion, bowel obstruction, perforation, positive margin, and inadequately sampled lymph nodes, according to National Comprehensive Cancer Network (NCCN) guidelines [3-6].
MSI, the abnormal shortening or lengthening of DNA by 1–6 repeating base pair units, results from the inactivation of the DNA mismatch repair (MMR) system and is found in approximately 15% of CCs [7]. Thus, MMR status is an important factor to consider when deciding whether to use adjuvant chemotherapy in patients with stage II CC [8]. According to previous studies, CC patients with MSI-high (MSI-H) tumors have a more favorable prognosis than those with microsatellite stable (MSS) tumors [9-11]. In addition, patients with MSI-low (MSI-L) or MSS tumors exhibited improved outcomes with 5-FU-based adjuvant chemotherapy, while adjuvant treatment was seemingly detrimental for patients with MSI-H stage II CC [10].
Recently, the European Society for Medical Oncology (ESMO) subdivided high-risk stage II CC into high-risk (T4, <12 lymph nodes or multiple risk factors) and intermediate-risk (lymphatic invasion, perineural invasion, vascular invasion, histologic grade 3, obstruction, or carcinoembryonic antigen [CEA] >5 ng/mL) groups. In addition, they recommended adjuvant FOLFOX or CAPOX for high-risk stage II CC regardless of MMR status and 5-FU or capecitabine chemotherapy alone for intermediate-risk stage II CC with MSS [12]. However, there are discrepancies in the chemotherapy recommendations for high- and intermediate-risk stage II CC between the ESMO and NCCN guidelines [6].
Accordingly, the present study evaluated the clinical implications of adjuvant chemotherapy for high-risk and intermediate-risk stage II CC according to the NCCN and ESMO guidelines. We also investigated the prognostic impact of clinicopathologic features, including MSI status, in patients with stage II CC.
Methods
Ethical statements: This study was approved by the Institutional Review Board (IRB) of Kyungpook National University Chilgok Hospital (IRB No: 2017-11-009), and the requirement for informed consent was waived.
1. Patients and treatment
This study retrospectively reviewed 5,774 patients who were diagnosed with CC and underwent curative surgical resection at Kyungpook National University Chilgok Hospital between January 2011 and December 2019. The patients were enrolled according to the following criteria: (1) pathologically diagnosed with primary CC; (2) stage II CC based on the 7th edition of the American Joint Committee on Cancer staging system [13]; (3) intermediate- and high-risk features [12]; and (4) available test results for MMR status. A total of 286 patients met all of these criteria and were included in the study (Fig. 1). Patient records were also reviewed for data on their medical history, age, sex, adjuvant chemotherapy regimen, surgical methods, and pathologic results.

Flow diagram of patient selection. CC, colon cancer; MMR, mismatch repair; MSI, microsatellite instability; MSI-H, MSI-high; dMMR, deficient MMR; MSI-L, MSI-low; MSS, microsatellite stable; pMMR, proficient MMR.
Adjuvant chemotherapy was started 3 to 6 weeks after surgery. In the case of capecitabine monotherapy (capecitabine of 1,250 mg/m2 twice a day, day [D] 1–D14) and CAPOX therapy (oxaliplatin of 130 mg/m2, D1 and capecitabine of 1,000 mg/m2 twice a day, D1–D14), the patients received chemotherapy every 3 weeks for 24 weeks [14,15]. In the case of FOLFOX therapy (oxaliplatin of 85 mg/m2, D1; leucovorin of 400 mg/m2, D1; 5-FU of 400 mg/m2 bolus, D1; and 5-FU of 2,400 mg/m2 continuous, D1–D2), the patients received 12 cycles of chemotherapy every 2 weeks [16,17]. The 5-FU/leucovorin regimen (5-FU of 425 mg/m2 and leucovorin of 20 mg/m2, D1–D5) was administered every 4 weeks for six cycles. Dose modifications were performed according to predefined guidelines based on toxicity responses [18]. Observation without adjuvant therapy was also an option for patients who were elderly or patients with an Eastern Cooperative Oncology Group performance status of ≥3.
2. Definition of high-risk stage II disease by National Comprehensive Cancer Network guidelines
For patients with MSS, stage II disease was classified as high risk if they exhibited at least one of the poor prognosis features, while all patients with MSI-H were excluded from the high-risk group [6].
3. Definition of intermediate- and high-risk stage II disease by European Society for Medical Oncology guidelines
Patients with stage II disease were classified as intermediate risk if they exhibited one of the poor prognosis features except for a T4 tumor or inadequately sampled lymph nodes (<12 lymph nodes). Patients with stage II disease were classified as high risk if they exhibited a T4 tumor, including perforation and/or inadequately sampled lymph nodes or several intermediate-risk factors [12].
4. Determination of mismatch repair status
MSI was evaluated based on immunohistochemistry (IHC) analysis of the expression of MMR proteins (MLH1, MSH2, MSH6, and PMS2) or by molecular MSI testing based on a polymerase chain reaction (PCR) assay [19]. IHC for MMR protein expression was performed on whole sections using an automatic immunostainer (BenchMark XT, Ventana Medical Systems, Tucson, AZ, USA) according to the manufacturer’s instructions. Primary monoclonal antibodies against MLH1 (clone M1, prediluted, Ventana Medical Systems), MSH2 (clone G219, 1:100, Cellmark, Rocklin, CA, USA), MSH6 (clone 44, prediluted, Ventana Medical Systems), and PMS2 (clone mrq-28, 1:200, Cellmark), and an ultraView Universal DAB kit (Ventana Medical Systems) were applied to 4-µm-thick 10% formalin-fixed tissue sections. Tumors displaying loss of expression of one or more MMR proteins were considered deficient MMR (dMMR), whereas those with intact MMR proteins were classified as proficient MMR (pMMR). Meanwhile, molecular MSI testing used a panel consisting of five markers (D5S346, BAT26, BST25, D17S250, and D2S123). The amplified PCR products were analyzed using a Model 3500×L Genetic Analyzer (Thermo Fisher Scientific, Seoul, Korea). A locus was determined to be unstable if unequivocal instabilities were observed in the tumor sample in comparison with paired normal DNA from the same patient. The MSI was graded as high (MSI-H) when two or more markers were unstable, low (MSI-L) when one marker was unstable, and stable (MSS) when all markers were stable.
5. Statistical analysis
Descriptive statistics are reported as proportions and medians. Categorical variables were evaluated using chi-square and Fisher exact tests, as appropriate. Disease-free survival (DFS) was measured from the date of surgery to the date of tumor recurrence or all-cause mortality. Overall survival (OS) was calculated from the date of surgery to that of all-cause mortality. Data were censored if patients were free of recurrence or were alive at the last follow-up. The Kaplan-Meier method was used to estimate DFS and OS. The survival curves were compared using a log-rank test according to MMR status or adjuvant chemotherapy. Multivariate survival analyses were performed using the Cox proportional hazard regression model. The hazard ratio and 95% confidence interval were estimated for each factor. Statistical significance was set at p<0.05. Statistical analyses were performed using IBM SPSS ver. 21.0 for Windows (IBM Corp., Armonk, NY, USA).
Results
1. Patient and tumor characteristics
The patient and tumor characteristics are summarized in Table 1. The median age was 70 years (range, 25–88 years) at the time of diagnosis, and 153 patients (53.5%) were male. According to the MMR status results, 54 patients (18.9%) were identified as MSI-H/dMMR. The primary tumors were located in the ascending colon in 100 patients (35.0%), transverse colon in 56 patients (19.6%), and descending colon in 130 patients (45.5%). Right-sided CC was observed in 147 patients (51.4%), and left-sided CC was observed in 139 patients (48.6%). The frequencies of intermediate- and high-risk features were as follows: T4 tumor (n=51, 17.8%), fewer than 12 lymph nodes examined (n=28, 9.8%), obstruction (n=22, 7.7%), perforation (n=3, 1.0%), high-grade tumor (n=25, 8.7%), perineural invasion (n=162, 56.6%), and lymphovascular invasion (n=184, 64.3%). Among the 286 eligible patients, 201 (70.3%) received adjuvant therapy. Among these 201 patients, 99 (49.3%) received capecitabine alone, four (2.0%) received 5-FU/leucovorin, 95 (47.3%) received FOLFOX, and three (1.5%) received CAPOX as adjuvant chemotherapy. Among the 86 patients with intermediate-risk and MSI-L/MSS or pMMR, 53 (61.6%) received adjuvant therapy. Among these 53 patients, 28 (52.8%) received either capecitabine alone or 5-FU/leucovorin in combination, and 25 (47.2%) received either FOLFOX or CAPOX as adjuvant chemotherapy. The incidence of MSI-H/dMMR was higher with right-sided CC (n=41, 27.9%) and high-grade tumors (n=11, 44.0%).
2. Survival outcomes
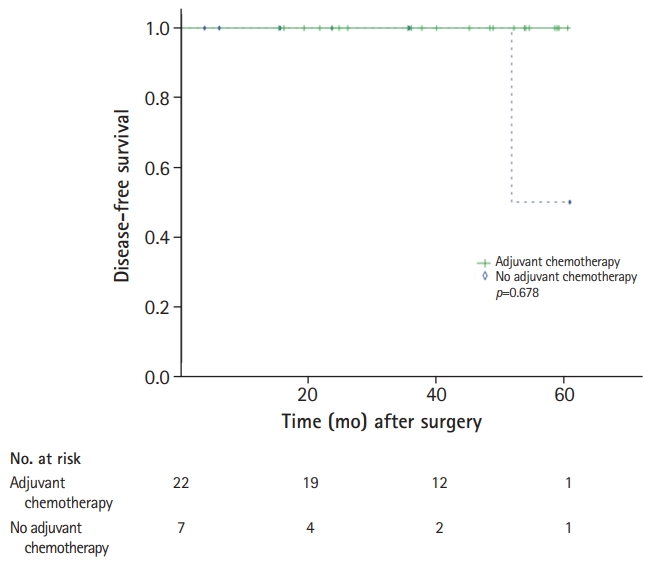
With a median follow-up duration of 36.0 months (range, 0.5–105.2 months), the estimated 3-year DFS and OS rates were 88.9% and 93.8%, respectively. During the analyses, 32 patients (11.2%) experienced disease relapse, and 19 patients (6.6%) died. Among the patients with MSI-H, only two experienced relapse, and only one died. According to ESMO guidelines, 115 patients (40.2%) were classified as intermediate risk and 171 (59.8%) as high risk (Table 1). The incidence of MSI-H/dMMR was higher among intermediate-risk patients (n=29, 25.2%) than among high-risk patients (n=25, 14.6%). For the intermediate-risk patients identified as MSI-L/MSS or pMMR (n=86), seven patients experienced a relapse and three patients died. Only one patients who received capecitabine as adjuvant chemotherapy experienced relapse and death, but none of the patients who received an oxaliplatin-containing regimen as adjuvant chemotherapy experienced either relapse or death. For the intermediate-risk patients identified as MSI-L/MSS or pMMR, adjuvant chemotherapy produced a significantly better DFS (p=0.002), yet had no impact on OS (p=0.176) (Fig. 2). The oxaliplatin-containing regimens were not associated with DFS or OS (Fig. 3). For the intermediate-risk patients identified as MSI-H/dMMR, only one patients who did not receive adjuvant chemotherapy experienced relapse and adjuvant chemotherapy showed no association with DFS (p=0.678) (Fig. 4).

Kaplan-Meier survival curves ffor (A) disease-free and (B) overall survival of patients with intermediate-risk stage II colon cancer and microsatellite instability-low/microsatellite stable according to adjuvant chemotherapy.

Kaplan-Meier survival curves for (A) disease-free and (B) overall survival of patients with intermediate-risk stage II colon cancer and microsatellite instability-low/microsatellite stable or proficient mismatch repair according to type of adjuvant chemotherapy.
3. Prognostic value of microsatellite instability and factors affecting survival outcomes
In the multivariate analysis including intermediate- and high-risk patients, a T4 tumor and adjuvant chemotherapy were both identified as independent prognostic factors for DFS (Table 2) and OS (Table 3).
Discussion
Accumulating data suggest that MMR status and clinicopathologic features are both important determinants in deciding whether to pursue adjuvant chemotherapy for patients with stage II CC. However, the use of adjuvant chemotherapy in intermediate-risk stage II patients remains debatable. Therefore, the present study investigated the clinical impact of adjuvant chemotherapy in a relatively large cohort of intermediate-risk stage II CC patients. As a result, the intermediate-risk patients identified as MSI-L/MSS or pMMR exhibited improved outcomes with adjuvant chemotherapy, but the addition of oxaliplatin showed no survival benefit. Thus, a further prospective randomized study is needed to explore the benefit of oxaliplatin in adjuvant therapy for MSI-L/MSS or pMMR intermediate-risk stage II patients. Meanwhile, the intermediate-risk patients with tumors identified as MSI-H/dMMR in the present study showed no statistically significant benefit from adjuvant chemotherapy.
Several guidelines suggest that certain clinicopathologic high-risk features may be predictive of benefit from adjuvant chemotherapy for patients with stage II CC [20]. According to NCCN guidelines, high-risk features include T4 tumors; poorly differentiated/undifferentiated histology; lymphovascular invasion; perineural invasion; tumor budding; bowel obstruction; lesions with localized perforations or close, indeterminate, or positive margins; and inadequately sampled lymph nodes (<12 nodes) [6]. Thus, for high-risk patients, adjuvant therapy can be considered in conjunction with patient/physician discussions personalized for each patient [3,21]. Meanwhile, ESMO guidelines propose both major prognostic parameters (pathological [p] T4 stage including perforations and lymph node sampling <12) and minor prognostic parameters (high-grade tumor, vascular invasion, lymphatic invasion, perineural invasion, tumor presentation with obstruction, and high preoperative CEA levels) [12]. For intermediate-risk patients (non-MMR/MSI and any risk factor except pT4 or <12 lymph nodes assessed), 6 months of 5-FU treatment is recommended [12]. However, most studies addressing the role of adjuvant treatment in high-risk stage II settings have been retrospective or unplanned analyses [22]. Moreover, the limitations of these studies are the biologic heterogeneity of the various factors and the lack of an unequivocal definition of clinicopathologic conditions [23]. Nevertheless, the current findings confirm a significant survival benefit for MSI-L/MSS or pMMR intermediate-risk stage II CC patients treated with adjuvant therapy when compared to patients not receiving adjuvant therapy. Furthermore, the current analyses excluded high-risk patients with pT4 and/or <12 lymph nodes and several intermediate-risk factors known as robust risks of relapse after CC resection [4]. The current findings also narrow the indications for adjuvant chemotherapy and may help in establishing appropriate treatment strategies and disease prognosis for patients with stage II CC.
Besides clinicopathologic factors, selection of the adjuvant regimen varies depending on clinical considerations such as the patient’s performance status, comorbidities and tolerance, and physician/patient preference [18]. In the current study, no survival benefits were noted when oxaliplatin was added to the adjuvant regimens for intermediate-risk stage II patients identified as MSI-L/MSS or pMMR. This result is consistent with those of previous studies. The results from a recent post-hoc exploratory analysis of the MOSAIC trial showed no significant DFS benefit of FOLFOX when compared with infusional 5-FU/leucovorin [5,24]. The NSABP-07 trial also showed no benefit from oxaliplatin-containing regimens [25]. Of note, the definition of high-risk differs among such studies, and no prospective trial has yet compared oxaliplatin-based therapy in intermediate-risk patients [20]. However, the current results clearly question the use of oxaliplatin in adjuvant chemotherapy for patients with intermediate-risk stage II CC. Therefore, further large-scale studies are required to identify the features predictive of benefit from oxaliplatin-based therapy in MSI-L/MSS or pMMR intermediate-risk stage II CC.
Interestingly, the present study demonstrated that MSI-H/dMMR status was associated with better prognosis, but this status did not predict the benefit of adjuvant chemotherapy in intermediate-risk stage II CC patients. Thus, despite substantial evidence of MSI-H/dMMR as a prognostic marker of a more favorable outcome, the role of adjuvant treatment for stage II CC patients with MSI-H/dMMR status remains unclear [11]. Several studies have also reported that MSI-H/dMMR status may be a predictive marker of decreased benefit and possibly detrimental impact of adjuvant therapy with 5-FU alone in patients with stage II CC [9,10], whereas other recent studies revealed no association with adjuvant treatment, which is consistent with the survival results of the present study [26,27]. Thus, when taken together, the current observations on the association of MSI-H/dMMR status and impact of adjuvant chemotherapy would seem to offer meaningful information and a novel strategy for patient subgroups with different risks.
The use of adjuvant chemotherapy was found to correlate with better DFS in MSI-L/MSS or pMMR intermediate-risk stage II CC patients, thereby warranting further clarification of the role of adjuvant chemotherapy and benefit of oxaliplatin-containing regimens for MSI-L/MSS or pMMR intermediate-risk stage II CC patients after curative resection.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization: BWK, JHB, JGK; Data curation: DWB, EC, HJK, SYP, JSP, GSC; Formal analysis: BWK, JHB, JGK; Visualization: BWK; Writing-original draft: BWK, JHB, JGK; Writing-review & editing: BWK, JHB, JGK.