Safety of low-dose anticoagulation in extracorporeal membrane oxygenation using the Permanent Life Support System: a retrospective observational study
Article information
Abstract
Background
Bleeding and thrombosis are major complications associated with high mortality in extracorporeal membrane oxygenation (ECMO) management. Anticoagulant therapy should be adequate to reduce thrombosis. However, related studies are limited.
Methods
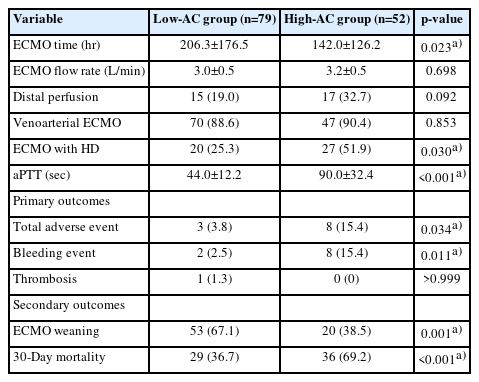
We retrospectively reviewed all patients supported with ECMO at a single institution between January 2014 and July 2022 and included those on all types of ECMO using the Permanent Life Support System. Patients were classified into two groups according to their measured mean activated partial thromboplastin time (aPTT) during ECMO management: a high-anticoagulation (AC) group (aPTT, ≥55 seconds; n=52) and a low-AC group (aPTT, <55 seconds; n=79). The primary outcome was thrombotic or bleeding events during ECMO.
Results
We identified 10 patients with bleeding; significantly more of these patients were in the high-AC group (n=8) than in the low-AC group (15.4% vs. 2.5%, p=0.01). However, thrombus events and oxygenator change-free times were not significantly different between the two groups. Four patients in the high-AC group died of bleeding complications (brain hemorrhage, two; hemopericardium, one; and gastrointestinal bleeding, one). One patient in the low-AC group developed a thrombus and died of ECMO dysfunction due to circuit thrombosis.
Conclusion
Heparin did not significantly improve thrombotic outcomes. However, maintaining an aPTT of ≥55 seconds was a significant risk factor for bleeding events, especially those associated with mortality.
Introduction
In extracorporeal membrane oxygenation (ECMO) management, anticoagulation (AC) is essential to prevent clotting in the ECMO circuit. According to the Extracorporeal Life Support Organization (ELSO) guidelines, the recommended activated partial thromboplastin time (aPTT) target in AC management during ECMO is 1.5 to 2.5 times the baseline aPTT of patients [1]. However, this recommended target is not based on randomized controlled trials, and references regarding target aPTT during ECMO management are lacking.
Some studies have sought to identify adequate AC strategies for ECMO [2-6]. These studies indicated that AC management, especially with heparin and aPTT maintenance over 1.5 times patients’ baseline aPTT, did not improve the results of thrombotic events or mortality and caused more bleeding complications. However, the authors divided their cohort according to the patients’ target aPTT and not the actual aPTT levels. Hence, a discrepancy may exist between the assigned group of patients and their actual measured aPTT values. For example, patients with disseminated intravascular coagulation can maintain high aPTT levels; however, if their target aPTT was <60 seconds, they would be assigned to the low-aPTT group rather than the high-aPTT group. To control this discrepancy, we divided patients according to their actual measured aPTT values, not their target aPTT and conducted an analysis to identify adequate AC strategies during ECMO.
Methods
Ethical statements: This study was approved by the Institutional Review Board (IRB) of Keimyung University Dongsan Medical Center (IRB No: 2022-12-038), and the requirement for informed consent was waived owing to the retrospective nature of the study.
1. Study design
We retrospectively reviewed all the patients who received ECMO at a single institution between January 2014 and July 2022. The patients were identified using a prospectively maintained database of all patients who received ECMO at our institution. Patients undergoing all types of ECMO (venovenous or venoarterial) using the Permanent Life Support (PLS) system (Maquet, Rastatt, Germany) were included. We excluded patients who were under 18 years of age, died in the first 24 hours of ECMO initiation, received other AC therapies (e.g., nafamostat and argatroban), underwent cardiovascular surgery, had coronavirus disease 2019 pneumonia, had heparin contraindications, had active bleeding during the first 24 hours of ECMO initiation, and were transferred to other hospitals.
AC management during ECMO was reviewed. Furthermore, we divided the patients into two groups according to their measured mean aPTT during ECMO: a high-AC group (aPTT, ≥55 seconds) and a low-AC group (aPTT, <55 seconds) (Fig. 1). Considering the loading dose of heparin at ECMO initiation, we did not include the first measured aPTT after starting ECMO in the calculation of the mean aPTT. We collected aPTT data at least 6 hours after ECMO initiation.
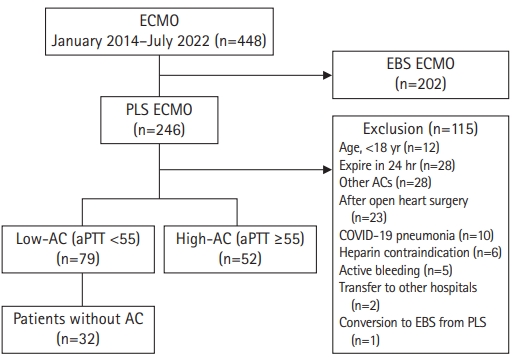
Patient selection and study cohorts. ECMO, extracorporeal membrane oxygenation; EBS, emergency bypass system; PLS, permanent life support; AC, anticoagulation; COVID-19, coronavirus disease 2019; aPTT, activated partial thromboplastin time (sec).
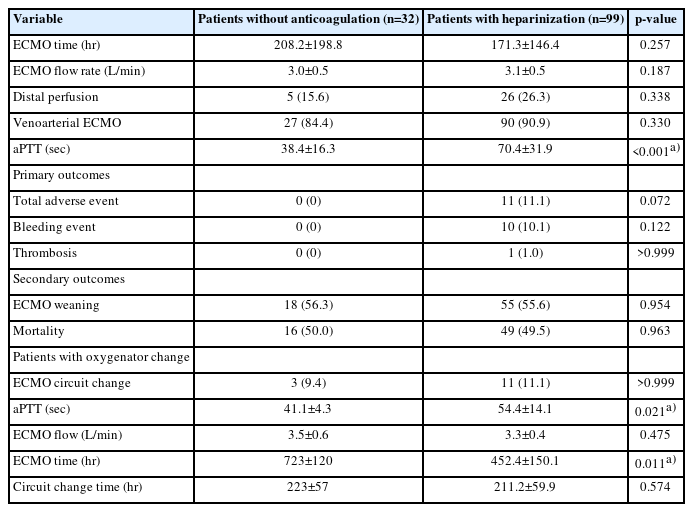
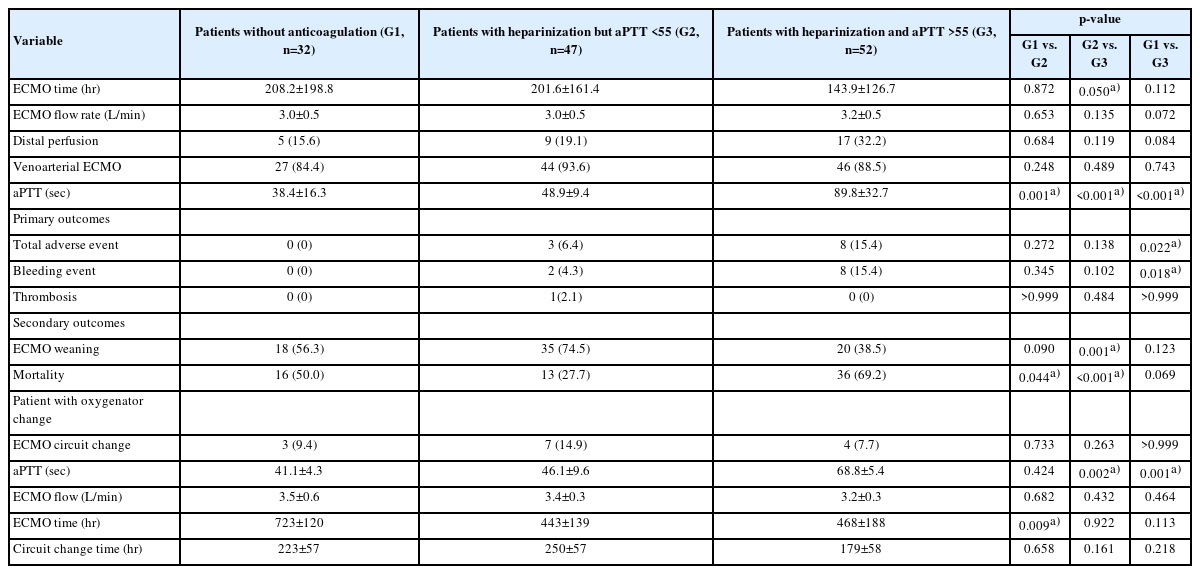
For the subgroup analysis, we further divided the patients into three groups (patients without AC, patients with heparinization and mean aPTT of <55 seconds, and patients with heparinization and mean aPTT of ≥55 seconds) to determine the effects of ECMO management without AC.
2. Data collection
Data regarding patient characteristics, ECMO indications, oxygenator change time, laboratory values, and ECMO support duration were collected from a prospectively maintained institutional ECMO database. We also retrospectively reviewed the patients’ charts to gather supplemental data and confirm the incidence of complications, relevant imaging findings, and outcomes throughout the hospital stay.
3. Outcomes
The primary outcomes were oxygenator change-free time and overall rate of hemorrhagic or thrombotic complications. Hemorrhagic complications included hemorrhagic cerebrovascular accidents, bleeding requiring intervention, and gastrointestinal bleeding requiring endoscopic evaluation. Ischemic cerebral vascular accidents, limb thrombosis, intracardiac thrombus, pulmonary embolism, and thrombosis formation in the ECMO circuit indicated thrombotic complications. According to the ELSO guidelines, the indications for oxygenator change after oxygenator failure are as follows: visible thrombus in the oxygenator, sudden increase in pressure difference before and after oxygenator change, continuously increasing oxygen demand, and decreasing capacity for carbon dioxide washout [1]. Secondary outcomes included the rate of ECMO weaning and overall in-hospital mortality.
4. Extracorporeal membrane oxygenation system and anticoagulation
Patients started on ECMO at our institution received a 50 IU/kg bolus of heparin before peripheral cannulation unless they were coagulopathic. None of the patients underwent heparin reversal with protamine treatment. Bedside peripheral cannulation was performed using the over-the-wire Seldinger technique in all patients in the final cohort. All arterial cannulas measured 15–17 French (Fr; HLS Cannula, Maquet), with 15 Fr used most frequently, whereas the venous cannulas measured 23 Fr (HLS Cannula, Maquet). The patients were monitored in the intensive care unit by a multidisciplinary team. Laboratory tests were performed twice daily as a standard of care, but the frequency was increased as clinically indicated.
5. Statistical analysis
Categorical variables, presented as numbers (percentages), were compared using Pearson chi-square test, whereas continuous variables, presented as means and standard deviations, were compared using the independent t-tests in the main analysis and using analysis of variance in the subgroup analysis. Oxygenator change-free time was calculated using the Kaplan-Meier method and compared using log-rank tests. All statistical data were analyzed using IBM SPSS ver. 20 (IBM Corp., Armonk, NY, USA). Furthermore, the p-values of <0.05 were considered statistically significant.
Results
1. Patient characteristics
Fig. 1 depicts patient eligibility for this study. Of the 246 adult patients who received PLS-ECMO, 115 (46.7%) were excluded. In the final cohort, 79 (60.3%) and 52 patients (39.7%) were classified into the low-AC and high-AC groups, respectively. In the low-AC group, 32 patients were treated without AC.
Table 1 shows the patient characteristics of the two groups. Patient age was similar between the low-AC group (60.3±12.1 years) and high-AC group (61.38±12.9 years) (p=0.67). Regarding the laboratory results obtained immediately before ECMO initiation, total bilirubin was significantly different between the low-AC and high-AC groups (1.04±0.82 mg/dL vs. 1.74±2.00 mg/dL, respectively; p=0.007). In contrast, baseline aPTT before ECMO initiation (31.9±14.9 in the low-AC group vs. 30.5±13.1 in the high-AC group; p=0.88) and other values including comorbidities were not significantly different between the two groups.
2. Results of extracorporeal membrane oxygenation management
Of the total cohort, 117 patients (89.3%) received venoarterial ECMO (VA-ECMO). Regarding clinical outcomes (Table 2), the low-AC group had a significantly longer ECMO management time than the high-AC group (206.3±176.5 hours vs. 142.0±126.2 hours, p=0.02) but had a significantly lower mean aPTT during this time (44.0±12.2 seconds vs. 90.0±32.4 seconds, p<0.001). However, there were significantly more patients undergoing ECMO with hemodialysis in the high-AC group than in the low-AC group (51.9% vs. 25.3%, p=0.03).
3. Primary and secondary outcomes
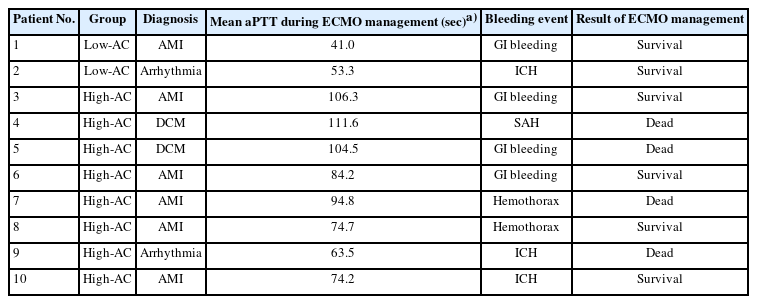
In the primary outcomes (Table 2), 10 major bleeding events occurred in both groups, with two in the low-AC group and eight in the high-AC group; however, the incidence of bleeding events was significantly higher in the high-AC group (15.4% vs. 2.5%, p=0.01). In the low-AC group, the bleeding events resulted from gastrointestinal bleeding and ECMO catheterization. These two patients survived and were eventually discharged from the hospital. In the high-AC group, the bleeding events were caused by brain hemorrhage, intrathoracic bleeding, and gastrointestinal bleeding in three, two, and three patients, respectively; among them, four died (brain hemorrhage, two; intrathoracic bleeding, one; and gastrointestinal bleeding, one). Table 3 shows the details of bleeding complications.
One patient in the low-AC group developed a major thrombus, and the ratio of thrombus event occurrence was not significantly different between the two groups (p>0.99). The patient underwent VA-ECMO after cardiogenic shock from myocardial infarction, and aPTT was maintained at 30 to 40 seconds. Unfortunately, the patient died of ECMO dysfunction owing to circuit thrombosis.
The mean oxygenator change-free time was 223±57 hours in the low-AC group and 179±58 hours in the high-AC group, but the difference was not significant (p=0.22). Overall, 14 patients experienced oxygenator changes, 10 in the low-AC group and four in the high-AC group. In other patients, fibrin deposition and thrombus formation were not observed in the oxygenator after ECMO device removal. Kaplan-Meier analysis revealed no significant difference in the results of oxygenator change-free time between the two subgroups (Fig. 2). Regarding secondary outcomes, the low-AC group had a higher rate of ECMO weaning than the high-AC group (67.1% vs. 38.5%, p=0.001) and a lower 30-day mortality rate (36.7% vs. 69.2%, p<0.001).
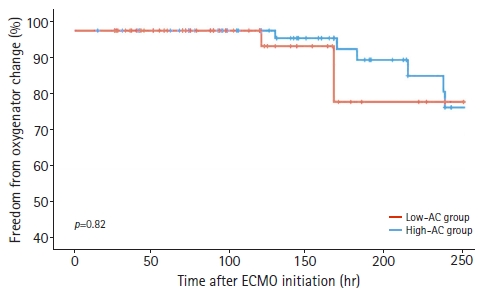
Oxygenator change-free time in low-AC and high-AC group (Kaplan-Meier analysis). AC, anticoagulation; ECMO, extracorporeal membrane oxygenation.
In the subgroup analysis (Tables 4, 5), patients who did not receive AC therapy showed no adverse events. The incidence of major adverse events was not significantly different between patients who did and did not receive AC therapy (Table 4). However, in the results comparing no AC and high AC (aPTT, ≥55 seconds), there were significantly more major bleeding events in the high-AC group than in the no-AC group (15.4% vs. 0%, p=0.02) (Table 5).
Discussion
This study has two strengths compared to other related reports. First, we classified patients according to their actual measured aPTT values and not according to their target aPTT levels. Second, this study only included ECMO cases using PLS because the types of ECMO machines and circuits are also important factors for bleeding or thrombus events.
The primary outcomes of this study were the effects of AC on hemorrhagic events, thrombotic events, and oxygenator change-free times. However, the optimal AC strategy for VA-ECMO management remains controversial. Various factors such as mechanical stress, blood-circuit interactions, and inflammatory cytokine release place patients at an increased risk of thrombotic and hemorrhagic complications [7-9]. However, in the present study, the lack of systemic AC and low-dose heparinization did not increase the thrombotic events in the ECMO circuit. Additionally, there was no significant difference in oxygenator change-free time (Fig. 2), and fibrin deposition and thrombus formation were not found in the oxygenators of patients who did not require oxygenator change after ECMO removal. Several studies have reported ECMO management with a lower target aPTT or without AC [10-16]. According to these reports, the lack of systemic AC did not increase thrombotic events in the ECMO circuit. Pump failure or circuit clots did not occur in the patients who did not receive AC therapy. Lamarche et al. [15] reported a low incidence of oxygenator failure (9%) in 32 patients who received VA-ECMO without AC, and similar results were reported in other reports [7,17]. The low risk of thromboembolic complications in ECMO with a lower aPTT or no heparin use may be related to improvements in the design of cannulas and oxygenators, while improving oxygenation and flow capabilities [18].
Regarding primary outcomes (Table 2), bleeding complications occurred significantly more frequently in the high-AC group. Among the eight patients with bleeding complications in the high-AC group, four died. No mortality was related to bleeding complications in the low-AC group. Previous reports on VA-ECMO support with low activated clotting time (140–160 seconds) or no AC therapy have similarly demonstrated a decreased incidence of major bleeding events and blood product transfusions, with no significant increase in thrombotic complications [15,19].
This study had several limitations. First, both venovenous ECMO and VA-ECMO were included. Thus, we could not exclude the effect on patient outcomes of the characteristics of each mode of ECMO according to the ECMO type. However, most of our patients (117 of 131, 89.3%) received VA-ECMO, and we believe that the mode of ECMO did not significantly affect the results of heparin use for thrombus, bleeding, and oxygenator survival. Second, patients were divided into two groups according to their measured aPTT levels. Hence, patients who were in poor condition, such as those with sepsis or multiple organ failure, tended to be assigned to the high-AC group because these patients usually had high aPTT levels, even though they had been managed with low target aPTT therapy or without AC. This study mainly aimed to determine the effect of aPTT levels on bleeding events, thrombosis events, and oxygenator survival time. Therefore, we set mortality and ECMO weaning rates as secondary rather than primary outcomes. However, a prospective randomized trial is required to confirm our findings.
Heparin use did not significantly improve the outcome of thrombotic events and the oxygenator change-free times during the ECMO period. Maintaining an aPTT of ≥55 seconds was a significant factor in the occurrence of bleeding events, especially those associated with mortality. Although our study does not warrant changing AC guidelines for ECMO because of its small sample size, the safety of using less or no heparin during ECMO is supported.
Notes
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Funding
None.
Author contributions
Conceptualization, Formal analysis: KS, JBK; Data curation, Investigation, Visualization: KS; Project administration, Supervision: JBK; Writing-original draft: KS; Writing-review & editing: KS, JBK.