Chemotherapy adherence is a favorable prognostic factor for elderly patients with multiple myeloma who are treated with a frontline bortezomib-containing regimen
Article information
Abstract
Background
Elderly patients with multiple myeloma (MM) are vulnerable to adverse events (AEs). This study evaluated adherence to chemotherapy and treatment outcomes in elderly patients treated with a frontline bortezomib (BTZ), melphalan, and prednisone (VMP) regimen and regimens without BTZ.
Methods
One-hundred and forty elderly patients who were diagnosed with MM from March 2007 to March 2015 were included in this retrospective study. To evaluate regimen adherence, patients who were treated with more than 4 cycles were assigned to the good adherence group.
Results
Among the 140 patients, 71 were treated with a frontline VMP and 69 with non-BTZ regimens. The median age was 71 years (range, 65-90 years). The VMP group showed a higher complete response rate than the non-BTZ group: 26.8% vs. 7.2%. More patients in the VMP group achieved ≥very good partial response (VGPR) and ≥PR. In the VMP group, 27 patients (38.0%) received less than 4 cycles. The VMP good adherence group showed a higher 3-year overall survival (OS) rate (70.9%) than the poor adherence group (60.2%, p=0.059). In the multivariate analysis, treatment with ≥4 cycles of VMP was a favorable factor for OS.
Conclusion
A good adherence to a frontline VMP regimen resulted in favorable long-term survival. Adequate management of AEs will be needed to achieve favorable outcomes in elderly patients with MM.
INTRODUCTION
For decades, frontline chemotherapy with melphalan and prednisone (MP) has been considered a standard regimen for elderly patients with multiple myeloma (MM) who are ineligible for high-dose therapy with hematopoietic stem cell transplantation [1]. However, several prospective, randomized, phase 3 studies comparing MP with MP plus novel agents, such as thalidomide (MPT), bortezomib (VMP), or lenalidomide (MPR) demonstrated that these novel agent combinations resulted in superior response rates and long-term survival, including time to progression (TTP), progression-free survival (PFS), and overall survival (OS) [2-5].
Bortezomib (BTZ) is a proteasome inhibitor that is active in relapsed, refractory, and newly diagnosed myeloma. In the Velcade as Initial Standard Therapy in Multiple Myeloma (VISTA) trial that compared VMP and MP regimens in patients who were not candidates for high-dose therapy, the proportions of patients who achieved a partial response (PR) or better were 71% in the VMP group and 35% in the MP group, and the corresponding complete response (CR) rates were 30% and 4%, respectively (p<0.001) [4]. After a median follow-up of 60.1 months (range, 0-74 months), there was a 31% reduced risk of death with VMP (median OS 56.4 months) vs. MP (median OS 43.1 months) [6].
Based on these results, BTZ was approved for patients with newly diagnosed MM, and VMP is now considered a standard of care in patients with newly diagnosed MM who are older than 65 years or who are not eligible for autologous stem cell transplantation (ASCT). However, caring for older adults with MM is particularly challenging because of comorbidities and frailty [7,8]. Older patients are prone to discontinue scheduled treatment due to side effects. One of the major toxicities of BTZ-containing regimens is peripheral neuropathy (PN), which was observed in 13% of patients in the VISTA trial [4]. Therefore, regimen compliance is compromised by these adverse events, and this may contribute to unfavorable outcomes.
This study retrospectively compared the outcomes of the VMP regimen with those of other regimens in patients with newly diagnosed myeloma who were ineligible for high-dose therapy. The current study also explored regimen adherence and primary regimen toxicity on the outcomes of elderly patients with MM.
MATERIALS AND METHODS
1. Data collection
This study retrospectively reviewed the treatment outcomes in 140 elderly patients with MM who were diagnosed from March 2007 to March 2015. Patients aged ≥65 years with a diagnosis of symptomatic myeloma and who were treated with frontline VMP or other regimens were included [9]. Patients aged <65 years and those who underwent ASCT, regardless of age, were excluded. This study was approved by the Institutional Review Board of each participating center.
2. Treatment
The frontline VMP regimen that was consisted of nine 6-week cycles of melphalan (9mg/m2) and prednisone (60 mg/m2) on days 1 to 4, in combination with BTZ(1.3mg/m2), by intravenous bolus or subcutaneous injection on days 1, 4, 8, 11, 22, 25, 29, and 32 during cycles 1 to 4, and on days 1, 8, 22, and 29 during cycles 5 to 9 [4,10]. Treatment with cyclophosphamide, thalidomide, and dexamethasone (CTD) was administered as previously described [11]. Patients who received BTZ treatment were administered acyclovir (200mg) twice daily, as herpes zoster prophylaxis. Patients also received trimethoprim/sulfamethoxazole during dexamethasone administration, as Pneumocystis jirovecii prophylaxis, and acetylsalicylic acid (100mg) to prevent deep vein thrombosis during thalidomide administration.
3. Assessments of response and toxicity
Response was assessed on day 1 of subsequent cycles. Response and progression were evaluated according to the International Myeloma Working Group uniform response criteria [12]. All adverse events were assessed on the days of each hospital visit and graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCICTCAE version 4.0) [13].
4. Statistical analysis
The categorical data were analyzed using a chi-square test, and continuous variables were compared using a Student’s t-test or analysis of variance (ANOVA). A logistic regression test was used to identify the factors that affected CR. OS was measured from the time of diagnosis to death or last follow-up. The groups treated with 4 cycles or more showed posttreatment responses, and the survival rates of these groups could be overinterpreted as associated with improved survival, when using the conventional method [14]. Thus, landmark plots with a landmark time of 160 days (4 chemotherapy cycles of VMP) were constructed, to illustrate the effects of chemotherapy cycles on OS. OS was analyzed using the Kaplan-Meier method and the log-rank test for comparison. Prognostic factors affecting OS were evaluated by a Cox regression model. Factors with p-values less than 0.1 in the univariate analyses were entered into the multivariate analyses, and p-values less than 0.05 were considered statistically significant. For statistical analyses, SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used.
RESULTS
1. Patient characteristics
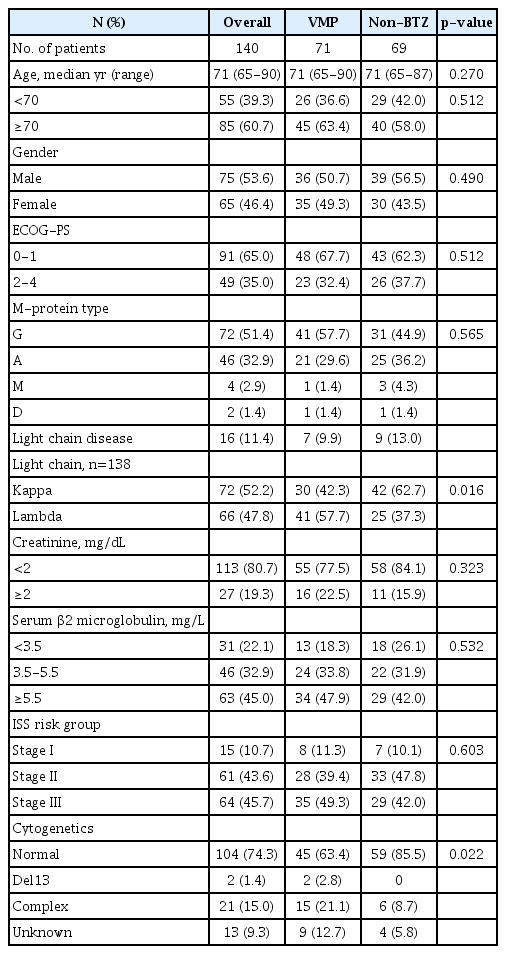
The data of 140 patients were collected and reviewed in the current study. The patient characteristics are summarized in Table 1. In brief, the median patient age was 71 years (range, 65-90 years), and 85 patients (60.7%) were 70 or older. International Staging System(ISS) risk groups included 15 (10.7%), 61 (43.6%), and 64 (45.7%) patients in stage I, II, and III, respectively. Twenty-seven patients (19.3%) had creatinine levels ≥2mg/dL. A frontline treatment regimen was administered using VMP in 71 patients (50.7%), and non-BTZ regimens in 69 (49.3%). Patient characteristics did not differ between VMP and non-BTZ groups, except for light chain type and complex cytogenetics. In the VMP and non-BTZ groups, 45 (63.4%) and 40 (58.0%) (p=0.512) patients were aged ≥70 years, respectively, and 23 (32.4%) and 26 (37.7%) (p=0.512) had Eastern Cooperative Oncology Group (ECOG) performance status ≥2. ISS stages did not differ between groups (p=0.603); however, the frequency of complex cytogenetics was higher in the VMP group.
2. Frontline therapy response and toxicity
In the VMP and non-BTZ groups, a median of 5 (range, 1-9) and 6 cycles (range, 1-77) of frontline treatment were administered, respectively (p=0.025) (Table 2). The VMP group showed a higher CR rate than the non-BTZ group: 26.8% vs. 7.2%. More patients in the VMP group achieved ≥very good partial response (VGPR) (50.7% vs. 18.8%, p<0.001) and ≥PR(73.2% vs. 55.1%, p=0.025). In the VMP group, PN was graded as 2 in 24 patients (33.8%) and 3 in 12 (16.9%), and the PN was higher in the VMP group than in the non-BTZ group (Table 3).
3. Compliance with frontline therapy
To evaluate regimen compliance, patients who were treated with 4 cycles or more were assigned to the good adherence group, because most responses occurred within 4 cycles. Furthermore, the health-related quality of life in elderly patients with MM who were treated with VMP differed by cycle 4, compared to those treated with MP [15,16].
Regimen compliance (≥4 cycles of frontline therapy) was slightly lower in the VMP group (44 patients, 62.0%) than in the non-BTZ group (52 patients, 75.4%), although this was not statistically significant (p=0.088). Among the 71 patients in the VMP group, less than 4 cycles were administered in 27 (38.0%). Bortezomib dose or schedule modification was performed in 75%(n=33/44) of patients treated with ≥4 cycles of VMP and in 89%(n=24/27) of patients treated with <4 cycles of VMP (p=0.153). The reasons for discontinuing the VMP regimen were as follows: nine patients had adverse events, 7 had insufficient responses (stable disease or progressive disease), 5 indicated preferences, 4 were lost to follow-up, and 2 died during treatment.
4. Survival outcomes
With a median follow-up duration of 14.0 months (range, 1.6-83.4 months), the 3-year OS rate of all patients was 52.9±6.3%. The median survival was 34.5 months in the non-BTZ group, but was not reached in the VMP group. The 3-year OS rates were 63.6±8.5% and 47.9±7.8% for VMP and non-BTZ regimens, respectively (Fig. 1). The patients who were treated with 4 cycles or more of VMP showed higher 3-year OS rates (70.9±10.1%) than those treated with less than 4 cycles of VMP (60.2±14.3%; p=0.059) in the landmark analysis (Fig. 2A). In the non-BTZ group, the 3-year OS rate was 53.3±9.5% for patients treated with ≥4 cycles and 35.6±13.4% for those treated with <4 cycles (p=0.052) in the landmark analysis (Fig. 2B). Patients who were treated with ≥4 cycles of VMP showed similar OS rates than those treated with ≥4 cycles of non-BTZ(p=0.134) (Fig. 3).
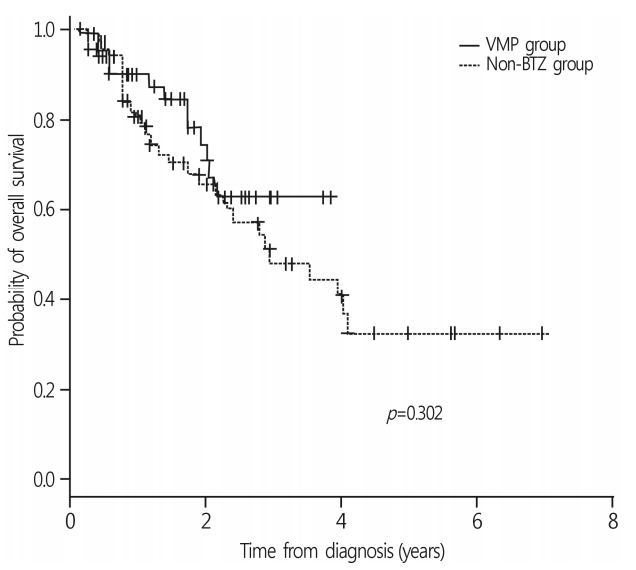
Overall survival rates between frontline VMP and nonbortezomib group. The 3-year OS rate of overall patients was 52.9±6.3%. The 3-year OS rates were 63.6±8.5% and 47.9±7.8% in VMP and non-BTZ regimens, respectively. VMP, bortezomib, melphalan, prednisone; OS, overall survival; BTZ, bortezomib.

OS rates according to the frontline chemotherapy cycles. (A) In VMP group, patients treated with VMP 4 cycles and more showed trend higher 3-year OS rate than those treated with VMP less than 4 cycles (70.9±10.1% vs. 60.2±14.3%; p=0.059). (B) In non-BTZ group, 3-year OS rate was 53.3±9.5% treated with ≥4 cycles and 35.6±13.4% in <4 cycles (p=0.052). The patients treated with VMP ≥4 cycles showed trend favorable OS rates compared to non-BTZ ≥4 cycles (p=0.061). OS, overall survival; VMP, bortezomib, melphalan, prednisone; BTZ, bortezomib.
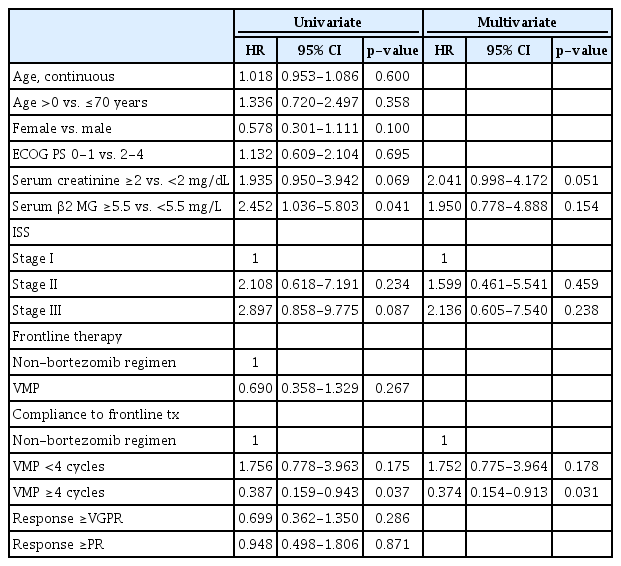
5. Factors affecting overall survival
In the univariate analyses, serum creatinine ≥2mg/dL, ISS stage, serum β2 microglobulin level ≥5.5 mg/L, and ≥4 cycles of the VMP regimen were entered in the multivariate model (Table 4). In the analysis, ≥4 cycles of the VMP regimen was a favorable factor for OS (hazard ratio [HR] 0.374, 95% confidence interval [CI] 0.154-0.913, p=0.031) (Table 4). Frontline VMP regimen and better responses (≥VGPR or ≥PR) did not significantly affect OS.
DISCUSSION
For more than 40 years, oral combination MP has been considered the standard of care for elderly patients with newly diagnosed MM[17]. Although MM remains an incurable disease, substantial survival gains in older patients with myeloma have been made in the last decade because of the availability of novel agents (i.e., thalidomide, lenalidomide, and bortezomib) [18-20].
The VMP regimen showed superior CR rates and TTP compared to MP in the VISTA trial [4,6]. The current study also confirmed the superior response rates with the frontline VMP regimen, compared to the non-BTZ regimen (Table 2). The 3-year-OS rates were similar to those in the VISTA trial: 63.6±8.5% for VMP and 47.9±7.8% for non-BTZ regimens; however, the median survival was 34.5 months in the non-BTZ group and was not reached in the VMP group. The initial survival disadvantages of the non-BTZ regimen were mitigated by subsequent BTZ-based regimens; therefore, statistical significance was not observed [21]. Among the 69 patients who received a frontline non-BTZ regimen, 26 were treated with second-line BTZ-based regimens.
Among the patients who received the frontline VMP regimen, those with good adherence to chemotherapy had better 3-year OS rates than those with poor adherence (Fig. 2A). Age-related organ function and metabolic changes affect the pharmacokinetics and pharmacodynamics of drugs and potentially increase toxicity, which can contribute to the poor tolerability of chemotherapeutic agents and the poorer outcomes seen in elderly patients with cancer [22,23]. Among the 27 patients (38.0%) who could not continue more than 4 cycles of therapy in the VMP group, 17 might have benefited from this regimen, excluding the 7 poor responders and the 2 treatment-related deaths. Proper managements of adverse events or dose adjustments can modify the disease course of such patients.
Palumbo et al. reported recently that continuous therapy with novel agents prolongs PFS and OS compared to fixed-dose therapy, without increasing chemotherapy-resistant relapse [24]. Although the beneficial effects of adherence and prolonged therapy were also observed for the non-BTZ regimens, our results are unable to support the study by Palumbo et al., because most patients who received non-BTZ regimens received traditional MP/CP, and the CR or VGPR rates of this group were around 20%. Nevertheless, the patients who were treated with non-BTZ regimens for ≥4 cycles showed favorable OS and tolerable adverse events; thus, it is worth considering the alternative of VMP for frail or vulnerable elderly patients with MM(Fig. 3) [7,25].
Quality of life (QOL) in MM is influenced by disease-related symptoms, treatment-related toxicity, and treatment response [26,27]. In addition to conventional response endpoints, QOL should be carefully evaluated in older adults with MM. Considering the frailty and vulnerability of elderly patients and successful salvage treatment with subsequent effective regimens, starting with tolerable agents could be a reasonable option for elderly patients who are unable to tolerate intensive combinations.
With respect to the limitations of this study, correlating poor adherence and side effects, analyzing dose intensities, and dose modification schedules are important, but these variables were not investigated thoroughly due to the retrospective nature of the study design. The frontline treatments were not randomly selected, so there could be selection bias between the groups with regards to baseline characteristics. Fluorescence in situ hybridization was not performed for most patients and the risk stratification was insufficient. Therefore, under treatment issues in the MP/CP regimens should be kept in mind when interpreting the data [28].
In conclusion, the frontline VMP regimen showed a higher response rate than the non-BTZ regimen, and good adherence resulted in favorable long-term survival. However, regimen adherence was compromised by a higher incidence of adverse events. Therefore, understanding the risk of toxicity and the adequate management of side effects are needed for favorable outcomes in patients receiving frontline BTZ-containing regimens.
Acknowledgements
H.J.C.*, S.S.K.* provided the acquisition, analysis and interpretation of data, drafted the article, revised it critically for important intellectual content; D.W.B., S.W.P., Y.J.L., S.K.S, H.S.L., W.S.L, J.H.L, S.H.K. performed the treatment, supplied the acquisition of data, and revised the manuscript; J.H.M. provided the conception and design of the study, revised the article critically for important intellectual content, and gave final approval of the version to be submitted.
*These authors contributed to this study as co-first authors.
Notes
No potential conflict of interest relevant to this article was reported.