Indexed in: ESCI, Scopus, PubMed,
PubMed Central, CAS, DOAJ, KCI
PubMed Central, CAS, DOAJ, KCI
FREE article processing charge

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 35(1); 2018 > Article
-
Original Article
Chemotherapy adherence is a favorable prognostic factor for elderly patients with multiple myeloma who are treated with a frontline bortezomib-containing regimen -
Hee-Jeong Cho1,*
 , Sang-Kyung Seo1,*
, Sang-Kyung Seo1,* , Dong Won Baek1, Sung-Woo Park1, Yoo-Jin Lee1, Sang-Kyun Sohn1, Ho-Sup Lee2, Won Sik Lee3, Ji Hyun Lee4, Sung Hyun Kim4, Joon-Ho Moon1
, Dong Won Baek1, Sung-Woo Park1, Yoo-Jin Lee1, Sang-Kyun Sohn1, Ho-Sup Lee2, Won Sik Lee3, Ji Hyun Lee4, Sung Hyun Kim4, Joon-Ho Moon1
-
Yeungnam University Journal of Medicine 2018;35(1):76-83.
DOI: https://doi.org/10.12701/yujm.2018.35.1.76
Published online: June 30, 2018
1Department of Hematology/Oncology, Kyungpook National University Hospital, Daegu, Korea
2Department of Hematology/Oncology, Kosin University Gospel Hospital, Busan, Korea
3Department of Hematology-Oncology, Inje University Pusan Baik Hospital, Busan, Korea
4Department of Hematology-Oncology, Dong-A University Hospital, Busan, Korea
- Corresponding Author: Joon-Ho Moon, Department of Hematology/Oncology, Kyungpook National University Hospital, School of Medicine, Kyungpook National University, 130, Dongdeok-ro, Jung-gu, Daegu 41944, Korea Tel: +82-53-200-6314, Fax: +82-53-426-2046 E-mail: jhmoon@knu.ac.kr
- *These authors contributed to this study as co-first authors.
• Received: April 30, 2018 • Revised: June 8, 2018 • Accepted: June 8, 2018
Copyright © 2018 Yeungnam University College of Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 6,199 Views
- 49 Download
- 3 Crossref
Abstract
-
Background
- Elderly patients with multiple myeloma (MM) are vulnerable to adverse events (AEs). This study evaluated adherence to chemotherapy and treatment outcomes in elderly patients treated with a frontline bortezomib (BTZ), melphalan, and prednisone (VMP) regimen and regimens without BTZ.
-
Methods
- One-hundred and forty elderly patients who were diagnosed with MM from March 2007 to March 2015 were included in this retrospective study. To evaluate regimen adherence, patients who were treated with more than 4 cycles were assigned to the good adherence group.
-
Results
- Among the 140 patients, 71 were treated with a frontline VMP and 69 with non-BTZ regimens. The median age was 71 years (range, 65-90 years). The VMP group showed a higher complete response rate than the non-BTZ group: 26.8% vs. 7.2%. More patients in the VMP group achieved ≥very good partial response (VGPR) and ≥PR. In the VMP group, 27 patients (38.0%) received less than 4 cycles. The VMP good adherence group showed a higher 3-year overall survival (OS) rate (70.9%) than the poor adherence group (60.2%, p=0.059). In the multivariate analysis, treatment with ≥4 cycles of VMP was a favorable factor for OS.
-
Conclusion
- A good adherence to a frontline VMP regimen resulted in favorable long-term survival. Adequate management of AEs will be needed to achieve favorable outcomes in elderly patients with MM.
- For decades, frontline chemotherapy with melphalan and prednisone (MP) has been considered a standard regimen for elderly patients with multiple myeloma (MM) who are ineligible for high-dose therapy with hematopoietic stem cell transplantation [1]. However, several prospective, randomized, phase 3 studies comparing MP with MP plus novel agents, such as thalidomide (MPT), bortezomib (VMP), or lenalidomide (MPR) demonstrated that these novel agent combinations resulted in superior response rates and long-term survival, including time to progression (TTP), progression-free survival (PFS), and overall survival (OS) [2-5].
- Bortezomib (BTZ) is a proteasome inhibitor that is active in relapsed, refractory, and newly diagnosed myeloma. In the Velcade as Initial Standard Therapy in Multiple Myeloma (VISTA) trial that compared VMP and MP regimens in patients who were not candidates for high-dose therapy, the proportions of patients who achieved a partial response (PR) or better were 71% in the VMP group and 35% in the MP group, and the corresponding complete response (CR) rates were 30% and 4%, respectively (p<0.001) [4]. After a median follow-up of 60.1 months (range, 0-74 months), there was a 31% reduced risk of death with VMP (median OS 56.4 months) vs. MP (median OS 43.1 months) [6].
- Based on these results, BTZ was approved for patients with newly diagnosed MM, and VMP is now considered a standard of care in patients with newly diagnosed MM who are older than 65 years or who are not eligible for autologous stem cell transplantation (ASCT). However, caring for older adults with MM is particularly challenging because of comorbidities and frailty [7,8]. Older patients are prone to discontinue scheduled treatment due to side effects. One of the major toxicities of BTZ-containing regimens is peripheral neuropathy (PN), which was observed in 13% of patients in the VISTA trial [4]. Therefore, regimen compliance is compromised by these adverse events, and this may contribute to unfavorable outcomes.
- This study retrospectively compared the outcomes of the VMP regimen with those of other regimens in patients with newly diagnosed myeloma who were ineligible for high-dose therapy. The current study also explored regimen adherence and primary regimen toxicity on the outcomes of elderly patients with MM.
INTRODUCTION
- 1. Data collection
- This study retrospectively reviewed the treatment outcomes in 140 elderly patients with MM who were diagnosed from March 2007 to March 2015. Patients aged ≥65 years with a diagnosis of symptomatic myeloma and who were treated with frontline VMP or other regimens were included [9]. Patients aged <65 years and those who underwent ASCT, regardless of age, were excluded. This study was approved by the Institutional Review Board of each participating center.
- 2. Treatment
- The frontline VMP regimen that was consisted of nine 6-week cycles of melphalan (9mg/m2) and prednisone (60 mg/m2) on days 1 to 4, in combination with BTZ(1.3mg/m2), by intravenous bolus or subcutaneous injection on days 1, 4, 8, 11, 22, 25, 29, and 32 during cycles 1 to 4, and on days 1, 8, 22, and 29 during cycles 5 to 9 [4,10]. Treatment with cyclophosphamide, thalidomide, and dexamethasone (CTD) was administered as previously described [11]. Patients who received BTZ treatment were administered acyclovir (200mg) twice daily, as herpes zoster prophylaxis. Patients also received trimethoprim/sulfamethoxazole during dexamethasone administration, as Pneumocystis jirovecii prophylaxis, and acetylsalicylic acid (100mg) to prevent deep vein thrombosis during thalidomide administration.
- 3. Assessments of response and toxicity
- Response was assessed on day 1 of subsequent cycles. Response and progression were evaluated according to the International Myeloma Working Group uniform response criteria [12]. All adverse events were assessed on the days of each hospital visit and graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCICTCAE version 4.0) [13].
- 4. Statistical analysis
- The categorical data were analyzed using a chi-square test, and continuous variables were compared using a Student’s t-test or analysis of variance (ANOVA). A logistic regression test was used to identify the factors that affected CR. OS was measured from the time of diagnosis to death or last follow-up. The groups treated with 4 cycles or more showed posttreatment responses, and the survival rates of these groups could be overinterpreted as associated with improved survival, when using the conventional method [14]. Thus, landmark plots with a landmark time of 160 days (4 chemotherapy cycles of VMP) were constructed, to illustrate the effects of chemotherapy cycles on OS. OS was analyzed using the Kaplan-Meier method and the log-rank test for comparison. Prognostic factors affecting OS were evaluated by a Cox regression model. Factors with p-values less than 0.1 in the univariate analyses were entered into the multivariate analyses, and p-values less than 0.05 were considered statistically significant. For statistical analyses, SPSS version 18.0 (SPSS Inc., Chicago, IL, USA) was used.
MATERIALS AND METHODS
- 1. Patient characteristics
- The data of 140 patients were collected and reviewed in the current study. The patient characteristics are summarized in Table 1. In brief, the median patient age was 71 years (range, 65-90 years), and 85 patients (60.7%) were 70 or older. International Staging System(ISS) risk groups included 15 (10.7%), 61 (43.6%), and 64 (45.7%) patients in stage I, II, and III, respectively. Twenty-seven patients (19.3%) had creatinine levels ≥2mg/dL. A frontline treatment regimen was administered using VMP in 71 patients (50.7%), and non-BTZ regimens in 69 (49.3%). Patient characteristics did not differ between VMP and non-BTZ groups, except for light chain type and complex cytogenetics. In the VMP and non-BTZ groups, 45 (63.4%) and 40 (58.0%) (p=0.512) patients were aged ≥70 years, respectively, and 23 (32.4%) and 26 (37.7%) (p=0.512) had Eastern Cooperative Oncology Group (ECOG) performance status ≥2. ISS stages did not differ between groups (p=0.603); however, the frequency of complex cytogenetics was higher in the VMP group.
- 2. Frontline therapy response and toxicity
- In the VMP and non-BTZ groups, a median of 5 (range, 1-9) and 6 cycles (range, 1-77) of frontline treatment were administered, respectively (p=0.025) (Table 2). The VMP group showed a higher CR rate than the non-BTZ group: 26.8% vs. 7.2%. More patients in the VMP group achieved ≥very good partial response (VGPR) (50.7% vs. 18.8%, p<0.001) and ≥PR(73.2% vs. 55.1%, p=0.025). In the VMP group, PN was graded as 2 in 24 patients (33.8%) and 3 in 12 (16.9%), and the PN was higher in the VMP group than in the non-BTZ group (Table 3).
- 3. Compliance with frontline therapy
- To evaluate regimen compliance, patients who were treated with 4 cycles or more were assigned to the good adherence group, because most responses occurred within 4 cycles. Furthermore, the health-related quality of life in elderly patients with MM who were treated with VMP differed by cycle 4, compared to those treated with MP [15,16].
- Regimen compliance (≥4 cycles of frontline therapy) was slightly lower in the VMP group (44 patients, 62.0%) than in the non-BTZ group (52 patients, 75.4%), although this was not statistically significant (p=0.088). Among the 71 patients in the VMP group, less than 4 cycles were administered in 27 (38.0%). Bortezomib dose or schedule modification was performed in 75%(n=33/44) of patients treated with ≥4 cycles of VMP and in 89%(n=24/27) of patients treated with <4 cycles of VMP (p=0.153). The reasons for discontinuing the VMP regimen were as follows: nine patients had adverse events, 7 had insufficient responses (stable disease or progressive disease), 5 indicated preferences, 4 were lost to follow-up, and 2 died during treatment.
- 4. Survival outcomes
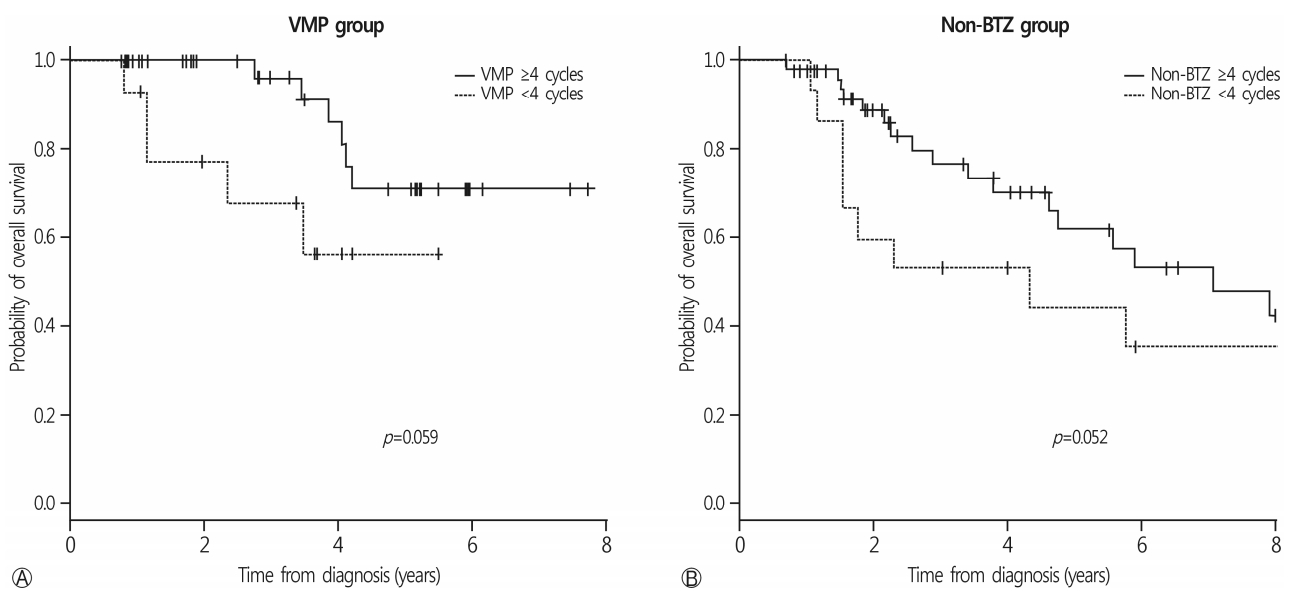
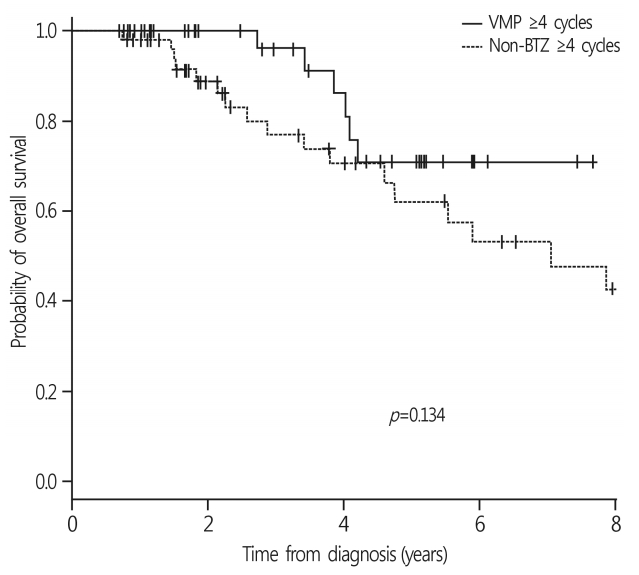
- With a median follow-up duration of 14.0 months (range, 1.6-83.4 months), the 3-year OS rate of all patients was 52.9±6.3%. The median survival was 34.5 months in the non-BTZ group, but was not reached in the VMP group. The 3-year OS rates were 63.6±8.5% and 47.9±7.8% for VMP and non-BTZ regimens, respectively (Fig. 1). The patients who were treated with 4 cycles or more of VMP showed higher 3-year OS rates (70.9±10.1%) than those treated with less than 4 cycles of VMP (60.2±14.3%; p=0.059) in the landmark analysis (Fig. 2A). In the non-BTZ group, the 3-year OS rate was 53.3±9.5% for patients treated with ≥4 cycles and 35.6±13.4% for those treated with <4 cycles (p=0.052) in the landmark analysis (Fig. 2B). Patients who were treated with ≥4 cycles of VMP showed similar OS rates than those treated with ≥4 cycles of non-BTZ(p=0.134) (Fig. 3).
- 5. Factors affecting overall survival
- In the univariate analyses, serum creatinine ≥2mg/dL, ISS stage, serum β2 microglobulin level ≥5.5 mg/L, and ≥4 cycles of the VMP regimen were entered in the multivariate model (Table 4). In the analysis, ≥4 cycles of the VMP regimen was a favorable factor for OS (hazard ratio [HR] 0.374, 95% confidence interval [CI] 0.154-0.913, p=0.031) (Table 4). Frontline VMP regimen and better responses (≥VGPR or ≥PR) did not significantly affect OS.
RESULTS
- For more than 40 years, oral combination MP has been considered the standard of care for elderly patients with newly diagnosed MM[17]. Although MM remains an incurable disease, substantial survival gains in older patients with myeloma have been made in the last decade because of the availability of novel agents (i.e., thalidomide, lenalidomide, and bortezomib) [18-20].
- The VMP regimen showed superior CR rates and TTP compared to MP in the VISTA trial [4,6]. The current study also confirmed the superior response rates with the frontline VMP regimen, compared to the non-BTZ regimen (Table 2). The 3-year-OS rates were similar to those in the VISTA trial: 63.6±8.5% for VMP and 47.9±7.8% for non-BTZ regimens; however, the median survival was 34.5 months in the non-BTZ group and was not reached in the VMP group. The initial survival disadvantages of the non-BTZ regimen were mitigated by subsequent BTZ-based regimens; therefore, statistical significance was not observed [21]. Among the 69 patients who received a frontline non-BTZ regimen, 26 were treated with second-line BTZ-based regimens.
- Among the patients who received the frontline VMP regimen, those with good adherence to chemotherapy had better 3-year OS rates than those with poor adherence (Fig. 2A). Age-related organ function and metabolic changes affect the pharmacokinetics and pharmacodynamics of drugs and potentially increase toxicity, which can contribute to the poor tolerability of chemotherapeutic agents and the poorer outcomes seen in elderly patients with cancer [22,23]. Among the 27 patients (38.0%) who could not continue more than 4 cycles of therapy in the VMP group, 17 might have benefited from this regimen, excluding the 7 poor responders and the 2 treatment-related deaths. Proper managements of adverse events or dose adjustments can modify the disease course of such patients.
- Palumbo et al. reported recently that continuous therapy with novel agents prolongs PFS and OS compared to fixed-dose therapy, without increasing chemotherapy-resistant relapse [24]. Although the beneficial effects of adherence and prolonged therapy were also observed for the non-BTZ regimens, our results are unable to support the study by Palumbo et al., because most patients who received non-BTZ regimens received traditional MP/CP, and the CR or VGPR rates of this group were around 20%. Nevertheless, the patients who were treated with non-BTZ regimens for ≥4 cycles showed favorable OS and tolerable adverse events; thus, it is worth considering the alternative of VMP for frail or vulnerable elderly patients with MM(Fig. 3) [7,25].
- Quality of life (QOL) in MM is influenced by disease-related symptoms, treatment-related toxicity, and treatment response [26,27]. In addition to conventional response endpoints, QOL should be carefully evaluated in older adults with MM. Considering the frailty and vulnerability of elderly patients and successful salvage treatment with subsequent effective regimens, starting with tolerable agents could be a reasonable option for elderly patients who are unable to tolerate intensive combinations.
- With respect to the limitations of this study, correlating poor adherence and side effects, analyzing dose intensities, and dose modification schedules are important, but these variables were not investigated thoroughly due to the retrospective nature of the study design. The frontline treatments were not randomly selected, so there could be selection bias between the groups with regards to baseline characteristics. Fluorescence in situ hybridization was not performed for most patients and the risk stratification was insufficient. Therefore, under treatment issues in the MP/CP regimens should be kept in mind when interpreting the data [28].
- In conclusion, the frontline VMP regimen showed a higher response rate than the non-BTZ regimen, and good adherence resulted in favorable long-term survival. However, regimen adherence was compromised by a higher incidence of adverse events. Therefore, understanding the risk of toxicity and the adequate management of side effects are needed for favorable outcomes in patients receiving frontline BTZ-containing regimens.
DISCUSSION
-
Acknowledgements
- H.J.C.*, S.S.K.* provided the acquisition, analysis and interpretation of data, drafted the article, revised it critically for important intellectual content; D.W.B., S.W.P., Y.J.L., S.K.S, H.S.L., W.S.L, J.H.L, S.H.K. performed the treatment, supplied the acquisition of data, and revised the manuscript; J.H.M. provided the conception and design of the study, revised the article critically for important intellectual content, and gave final approval of the version to be submitted.
- *These authors contributed to this study as co-first authors.
Fig. 1.Overall survival rates between frontline VMP and nonbortezomib group. The 3-year OS rate of overall patients was 52.9±6.3%. The 3-year OS rates were 63.6±8.5% and 47.9±7.8% in VMP and non-BTZ regimens, respectively. VMP, bortezomib, melphalan, prednisone; OS, overall survival; BTZ, bortezomib.
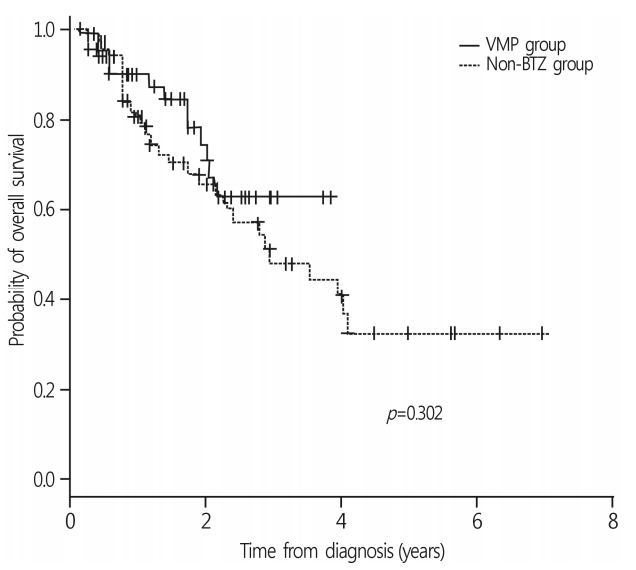

Fig. 2.OS rates according to the frontline chemotherapy cycles. (A) In VMP group, patients treated with VMP 4 cycles and more showed trend higher 3-year OS rate than those treated with VMP less than 4 cycles (70.9±10.1% vs. 60.2±14.3%; p=0.059). (B) In non-BTZ group, 3-year OS rate was 53.3±9.5% treated with ≥4 cycles and 35.6±13.4% in <4 cycles (p=0.052). The patients treated with VMP ≥4 cycles showed trend favorable OS rates compared to non-BTZ ≥4 cycles (p=0.061). OS, overall survival; VMP, bortezomib, melphalan, prednisone; BTZ, bortezomib.


Fig. 3.Comparison of OS rates in patients treated with 4 cycles and more. OS rates in patients treated with 4 cycles and more were not different between non-BTZ and VMP group (p=0.134). OS, overall survival; BTZ, bortezomib; VMP, bortezomib, melphalan, prednisone.


Table 1.Patient characteristics
Table 2.Treatment outcomes and response to frontline therapy
Table 3.Peripheral neuropathy
| Grade, n (%) | VMP | Non-BTZ regimen | p-value |
|---|---|---|---|
| 0 | 23 (32.4) | 62 (89.1) | <0.001 |
| 1 | 12 (16.9) | 2 (2.9) | |
| 2 | 24 (33.8) | 4 (5.8) | |
| 3 | 12 (16.9) | 1 (1.4) |
Table 4.Factors affecting overall survival
- 1. Gregory WM, Richards MA, Malpas JS. Combination chemotherapy versus melphalan and prednisolone in the treatment of multiple myeloma: an overview of published trials. J Clin Oncol 1992;10:334–42.ArticlePubMed
- 2. Palumbo A, Bringhen S, Caravita T, Merla E, Capparella V, Callea V, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet 2006;367:825–31.ArticlePubMed
- 3. Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood 2008;112:3107–14.ArticlePubMed
- 4. San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med 2008;359:906–17.ArticlePubMed
- 5. Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med 2012;366:1759–69.ArticlePubMed
- 6. San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Persistent overall survival benefit and no increased risk of second malignancies with bortezomib-melphalan-prednisone versus melphalan-prednisone in patients with previously untreated multiple myeloma. J Clin Oncol 2013;31:448–55.ArticlePubMed
- 7. Palumbo A, Bringhen S, Ludwig H, Dimopoulos MA, Bladé J, Mateos MV, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood 2011;118:4519–29.ArticlePubMed
- 8. Gay F, Palumbo A. Management of older patients with multiple myeloma. Blood Rev 2011;25:65–73.ArticlePubMed
- 9. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538–48.ArticlePubMed
- 10. Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, et al. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol 2011;12:431–40.ArticlePubMed
- 11. Yang DH, Kim YK, Sohn SK, Chung JS, Joo YD, Lee JH, et al. Induction treatment with cyclophosphamide, thalidomide, and dexamethasone in newly diagnosed multiple myeloma: a phase II study. Clin Lymphoma Myeloma Leuk 2010;10:62–7.ArticlePubMed
- 12. Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia 2006;20:1467–73.ArticlePubMedPDF
- 13. Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J Natl Cancer Inst 2014;106:pii: dju244.
- 14. Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol 1983;1:710–9.ArticlePubMed
- 15. Harousseau JL, Palumbo A, Richardson PG, Schlag R, Dimopoulos MA, Shpilberg O, et al. Superior outcomes associated with complete response in newly diagnosed multiple myeloma patients treated with nonintensive therapy: analysis of the phase 3 VISTA study of bortezomib plus melphalan-prednisone versus melphalan-prednisone. Blood 2010;116:3743–50.ArticlePubMed
- 16. Delforge M, Dhawan R, Robinson D Jr, Meunier J, Regnault A, Esseltine DL, et al. Health-related quality of life in elderly, newly diagnosed multiple myeloma patients treated with VMP vs. MP: results from the VISTA trial. Eur J Haematol 2012;89:16–27.ArticlePubMed
- 17. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6, 633 patients from 27 randomized trials. Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: an overview of 6,633 patients from 27 randomized trials. Myeloma Trialists' Collaborative Group. J Clin Oncol 1998;16:3832–42.ArticlePubMed
- 18. Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008;111:2516–20.ArticlePubMedPMC
- 19. Kumar SK, Dispenzieri A, Lacy MQ, Gertz MA, Buadi FK, Pandey S, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia 2014;28:1122–8.ArticlePubMedPDF
- 20. Pozzi S, Marcheselli L, Bari A, Liardo EV, Marcheselli R, Luminari S, et al. Survival of multiple myeloma patients in the era of novel therapies confirms the improvement in patients younger than 75 years: a population-based analysis. Br J Haematol 2013;163:40–6.ArticlePubMed
- 21. Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol 2010;28:2259–66.ArticlePubMed
- 22. Vestal RE. Aging and pharmacology. Cancer 1997;80:1302–10.ArticlePubMed
- 23. Sotaniemi EA, Arranto AJ, Pelkonen O, Pasanen M. Age and cytochrome P450-linked drug metabolism in humans: an analysis of 226 subjects with equal histopathologic conditions. Clin Pharmacol Ther 1997;61:331–9.ArticlePubMed
- 24. Palumbo A, Gay F, Cavallo F, Di Raimondo F, Larocca A, Hardan I, et al. Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. J Clin Oncol 2015;33:3459–66.ArticlePubMed
- 25. Larocca A, Palumbo A. How I treat fragile myeloma patients. Blood 2015;126:2179–85.ArticlePubMed
- 26. Delforge M, Minuk L, Eisenmann JC, Arnulf B, Canepa L, Fragasso A, et al. Health-related quality-of-life in patients with newly diagnosed multiple myeloma in the FIRST trial: lenalidomide plus low-dose dexamethasone versus melphalan, prednisone, thalidomide. Haematologica 2015;100:826–33.ArticlePubMedPMC
- 27. van der Poel MW, Oerlemans S, Schouten HC, van de Poll-Franse LV. Elderly multiple myeloma patients experience less deterioration in health-related quality of life than younger patients compared to a normative population: a study from the population-based PROFILES registry. Ann Hematol 2015;94:651–61.ArticlePubMed
- 28. Jung SH, Ahn JS, Yang DH, Cho MS, Kim JY, Ahn SY, et al. Oliguria as an early indicator of mortality risk in patients with multiple myeloma and renal impairment. Blood Res 2015;50:167–72.ArticlePubMedPMC
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Solomon Islands Oncology Unit: Sustainability in Terms of Outcomes
Dylan Bush, Mark Love, Hugo Bugoro, Nixon Panda
JCO Global Oncology.2024;[Epub] CrossRef - Real World Adherence to and Persistence With Oral Oncolytics in Multiple Myeloma: A Systematic Review and Meta-analysis
Abdallah Y. Naser, Richard Ofori-Asenso, Safaa Al Awawdeh, Sami Qadus, Hassan Alwafi, Danny Liew
Clinical Lymphoma Myeloma and Leukemia.2022; 22(10): 760. CrossRef - Oral therapy adherence and satisfaction in patients with multiple myeloma
Marine Solano, Etienne Daguindau, Cyril Faure, Pierre Loriod, Coline Pain, Anne-Cécile Maes, Pauline Marguet, Marie Kroemer, Anne Rumpler, Jean Fontan, Eric Deconinck, Samuel Limat, Anne-Laure Clairet
Annals of Hematology.2021; 100(7): 1803. CrossRef

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite