Indexed in: ESCI, Scopus, PubMed,
PubMed Central, CAS, DOAJ, KCI
PubMed Central, CAS, DOAJ, KCI
FREE article processing charge

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 37(3); 2020 > Article
-
Case report
Jejunogastric intussusception prone to misdiagnosis as gastric cancer -
Yong-Eun Park1
 , Sang-Woon Kim2
, Sang-Woon Kim2
-
Yeungnam University Journal of Medicine 2020;37(3):236-241.
DOI: https://doi.org/10.12701/yujm.2020.00038
Published online: April 6, 2020
1Department of Surgery, Yeungnam University Hospital, Daegu, Korea
2Department of Surgery, Yeungnam University College of Medicine, Daegu, Korea
- Corresponding author: Yong-Eun Park Department of Surgery, Yeungnam University Hospital, 170 Hyeonchung-ro, Nam-gu, Daegu 42415, Korea Tel: +82-53-620-3580 Fax: +82-53-624-1213 E-mail: cjschddnsrja@gmail.com
• Received: January 30, 2020 • Revised: March 30, 2020 • Accepted: March 31, 2020
Copyright © 2020 Yeungnam University College of Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 4,616 Views
- 57 Download
- 1 Crossref
Abstract
- The authors report a case of a 78-year-old female with a history of gastric surgery 35 years ago. She was initially misdiagnosed as gastric cancer bleeding and underwent an emergency laparotomy under the diagnosis of jejunogastric intussusception (JGI), 23 hours after the onset of symptoms. We also reviewed 116 JGI case reports and analyzed clinical features and outcomes. Compared to the past, diagnosis of JGI is easier with diagnostic examinations such as an endoscopy, computed tomography, and the upper gastrointestinal series. And a good prognosis can be expected with proper fluid resuscitation and surgical reduction, even if the symptoms persist more than 48 hours.
- Jejunogastric intussusception (JGI) occurs in only about 0.1% of gastro-enteric anastomosis and was first reported by Bozzi in 1914 [1,2]. In literature, approximately 300 cases have been reported and the interval from previous gastric surgery to JGI occurrence varies from days to decades [3,4]. Unawareness about the disease entity of JGI among clinicians causes an increase in mortality rate due to late diagnosis or misdiagnosis of JGI as gastric malignancy or bezoar. In the present case, we introduce a JGI patient who was misdiagnosed with gastric cancer bleeding and underwent surgical reduction 23 hours after onset of symptoms. We also reviewed previously published JGI case reports that were able to identify basic clinical information. We analyzed the clinical features and outcomes of 116 JGI case reports in the discussion section.
Introduction
- This study was approved by the Institutional Review Board of Yeungnam University Hospital (IRB No: 2020-01-004).
- A 78-years-old female visited the emergency room of the local hospital due to sudden and continuous epigastric pain from the previous evening. On abdominal computed tomography (CT), gastric distension with intragastric mass was found (Fig. 1) and old bloody drainage through Levin tube was seen. Under diagnosis of gastric cancer bleeding, she was transferred to the emergency room of Yeungnam University Hospital for further evaluation and management.
- At the time of arrival, the patient’s vital signs were stable and the color of drainage through Levin tube was yellowish. She had medical history of hypertension, diabetes mellitus, pneumonia (1 month back) and surgical history of distal gastrectomy (35 years back).
- Physical examination revealed a soft and mild distended abdomen with upper midline scar. There was a palpable mass and mild tenderness on left upper abdomen. There was no rebound tenderness or guarding sign. The laboratory examination showed leukocytosis (white blood cell count, 23.07 K/µL; neutrophil, 93.4%) and other findings including hemoglobin within normal range.
- The emergency medicine doctor in author’s center also misdiagnosed JGI as gastric cancer bleeding and consulted an internal medicine doctor for endoscopy. After 6 hours, internal medicine doctor performed endoscopy and recommended consulting general surgery department for gastric malignancy (Fig. 2). Initially, authors were not aware of the disease entity of JGI. However, continuous abdominal pain and leukocytosis without anemia could not be regarded as mere symptoms of gastric cancer. After reviewing findings of endoscopy and CT, we performed an emergency laparotomy under the diagnosis of JGI.
- The afferent loop was dilated and about 60 cm of efferent loop was intussuscepted into dilated stomach through gastro-jejunostomy site. After manual reduction and warm saline irrigation, there was no leading point and distal half of intussuscepted jejunum was not viable. Segmental resection of necrotic bowel was performed. And efferent loop was fixed to distal body of remnant stomach and mesentery of transverse colon to prevent recurrence of JGI. Due to postoperative pneumonia, patient was discharged on the 26th day of surgery.
Case
- The mean age of 100 JGI patients was 55 years (range, 27–83 years). There were 68 males (65.4%) and 36 females (34.6%). There were 83 cases (87.4%) that had a previous surgery for benign disease (80 cases of peptic ulcer, three cases of morbid obesity) and 12 cases (12.6%) had previous surgery for malignant disease (11 cases of gastric cancer, and one case of gall bladder cancer). The mean interval from previous surgery to diagnosis of JGI was 12.4±10.6 years (range, 2 days to 55 years; n=107) (Table 1). Shackman [5] classified JGI into three types according to the intussuscepted limb, type Ⅰ (antegrade intussusception of the afferent limb, 16%), type Ⅱ (retrograde intussusception of the efferent limb, 74%), type Ⅲ (combined form, 10%). Brynitz and Rubinstein [6] classified JGI into four types by adding type Ⅳ (intussusception through Braun’s side to side anastomosis). In our results, the percentage of each type was slightly different, but type Ⅱ was most common type as shown. Among 116 cases, there were five cases (4.3%) of type Ⅰ, 98 cases (84.5%) of type Ⅱ, five cases (4.3%) of type Ⅲ, and eight cases (6.9%) of type Ⅳ (Table 1). The author’s case was type Ⅱ, which is the most common type of JGI.
- The pathogenesis of JGI remains unclear. But it is thought that mechanical factors and functional factors affect the occurrence of JGI [7]. Functional factors include hyperperistalsis due to hyperacidity or spasm and retrograde peristalsis due to jejunitis or increased abdominal pressure [8]. Mechanical factors include postoperative adhesion, dilatation of bowel following gastrectomy, and long afferent loop or mesentery [9].
- There were 100 cases (86.2%) of JGI with acute symptoms, seven cases (6%) of JGI with chronic symptoms, and nine cases (7.8%) of JGI with acute on chronic symptoms. Most JGI shows acute manifestations, but some appear to be chronic and may change to acute if the diagnosis is delayed (Table 1). In the author’s case, patient complained of sudden and continuous epigastric pain and physical examination showed a palpable mass on the upper abdomen. Severe colicky epigastric pain, hematemesis, presence of a palpable mass, and vomiting are acute symptoms of JGI [10]. Of the 101 cases that mentioned specific symptoms, there were 89 cases (88.1%) with abdominal pain, 96 cases (95%) with vomiting, and 52 cases (51.5%) with hematemesis. Hematemesis (51.5%) and palpable mass (34.5%) were not as common as abdominal pain (88.1%) and vomiting (95%) (Table 1). The color of vomit might change from clear to coffee color with the progression of ischemia of intussuscepted limb. If clinicians know the disease entity of JGI, it becomes easier to diagnosis JGI with acute symptoms with endoscopy or CT. But if the intussusception of limb is intermittent and self-reversible, patient may complain of vague and chronic symptoms like recurrent bouts of epigastric area and nausea [11]. Diagnosis of JGI with chronic manifestation is difficult and can be performed by timely examination when the symptoms are present [10]. Of the 48 cases that mentioned laboratory findings, there were 32 cases (66.7%) with non-specific findings, five cases (10.4%) with leukocytosis, three cases (6.3%) with anemia, eight cases (16.7%) with anemia and leukocytosis (Table 1). Therefore, even if the laboratory finding is within normal limit, the diagnosis of JGI cannot be ruled out.
- Endoscopy and abdominal CT are diagnostic examinations of JGI [12,13]. During endoscopy, intussuscepted jejunum is visualized. And the nature of intussuscepted jejunum can be various from small intussuscepted jejunum with viable mucosa to bulky congestive mass according to the length of jejunum and degree of ischemia. On the axial and coronal view of CT, intussuscepted jejunal loop with entrapped mesenteric vessel and fat is seen in dilated stomach. Several signs such as claw sign on axial view and target sign on sagittal view are also helpful in the diagnosis of JGI [14]. Of the 79 cases with mention of diagnostic examinations, there were 46 cases (58.2%) endoscopy, 36 cases (45.6%) with CT, 26 cases (32.9%) with upper gastrointestinal (UGI) series, and 12 cases (15.2%) with ultrasonography. Although UGI series and ultrasonography were not as commonly performed diagnostic test as endoscopy and CT, but they also were useful in diagnosis of JGI. In most JGI cases before 1970, JGI was clinically diagnosed with reference to an abdominal X-ray or UGI series (Table 2).
- The treatment of choice for JGI is the surgical reduction of intussuscepted bowel with correction of dehydration and electrolyte disturbance [10]. Also, fixation of intussuscepted limb to adjacent tissues such as parietal wall, transverse colon mesentery, and stomach is required to prevent recurrence of JGI [14]. Authors fixed efferent loop to remnant stomach and transverse colon mesentery. In addition to manual reduction and fixation, bowel resection might be required according to viability of intussuscepted jejunum [15]. There were 82 cases (85.4%) with a surgical treatment. Among these cases, 41 cases (52.6%) had an additional bowel resection and 22 cases (32.4%) had fixation to adjacent tissue. There were six cases of recurrence after surgical treatment, of which two cases had a fixation procedure during previous surgery. Therefore, it is hard to say that the fixation can completely prevent the recurrence of JGI (Table 2).
- Endoscopic reduction of JGI is a possible option in the treatment of JGI through which general anesthesia and surgical complications such as pneumonia and wound infection can be avoided [16]. Nevertheless, endoscopic reduction of JGI has a significant risk of recurrence and should not be performed in situation where bowel ischemia or peritoneal irritation sign is suspected [17]. There were six cases (6.3%) of endoscopic reduction, of which two cases recurred. The recurrence rate of an endoscopic reduction (2 out of 6, 33.3%) was higher than the recurrence rate of a surgical reduction (6 out of 82, 7.3%) (Table 2).
- In this case, due to initial misdiagnosis, the surgical reduction was performed 23 hours after occurrence of sudden epigastric pain. It has been reported that if surgical treatment is performed within first 48 hours, the mortality rate of JGI patient can be about 10%, if not, may increase over 50% [18]. In our results, only 34 out of 100 acute JGI cases mentioned the duration of the symptoms. The mean duration of acute symptom was 45.7±43.4 hours and ranged 1 hour to 7 days (Table 1). The mortality rate was 8.3% (8 out of 96) and all mortality cases were reported before 1970s. Interestingly, all JGI case reports after 1970 had endoscopic or surgical reduction and had good prognosis regardless of the duration of the symptoms (Table 2, Fig. 3).
- If clinicians are aware of the disease entity of JGI, JGI is easily diagnosed by various examinations such as endoscopy, CT, and the UGI series. And a good prognosis can be expected even if the symptoms persist more than 48 hours, provided adequate fluid resuscitation and surgical reduction are performed.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Author contributions
Conceptualization: YEP, SWK; Data curation, Formal analysis: YEP, SWK; Writing-original draft, Writing-review & editing: YEP.
Notes
Fig. 1.Computed tomography findings of the jejunogastric intussusception patient. Both coronal (A) and axial view (B) show the intussuscepted jejunal loop (arrow) with the entrapped mesenteric vessel and fat (arrowhead) in the dilated stomach.
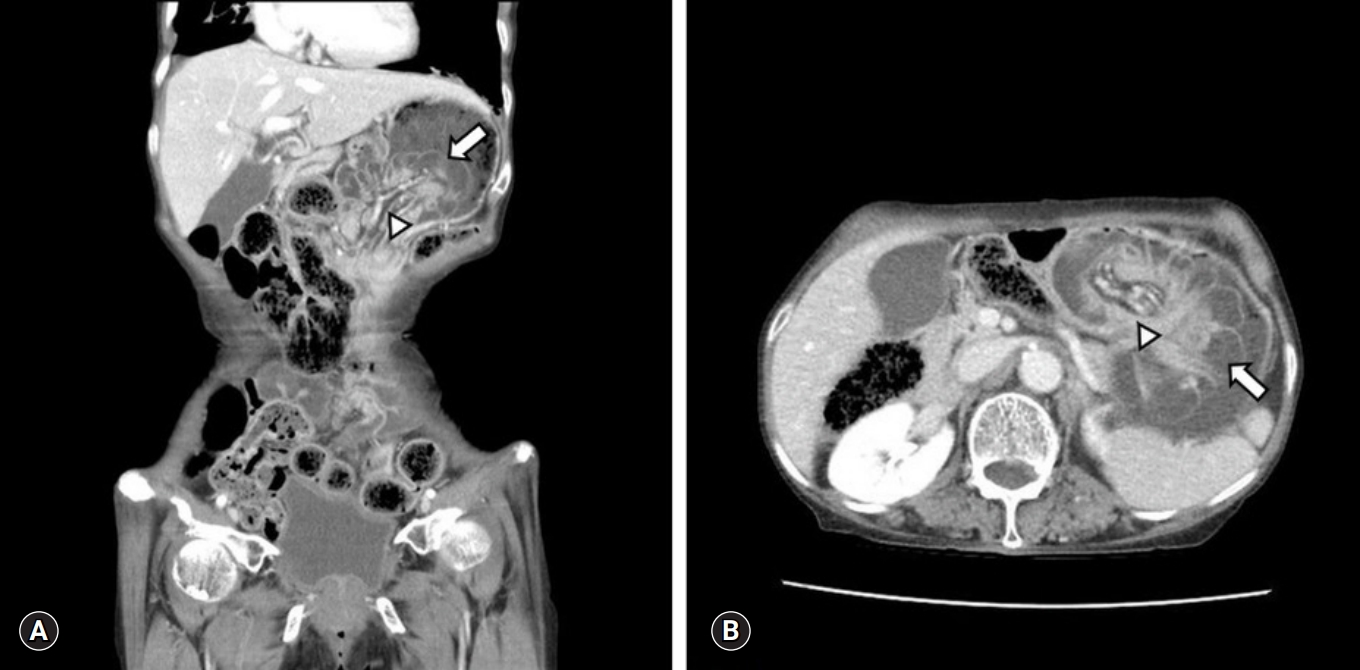

Fig. 2.Endoscopic findings of jejunogastric intussusception patient. (A) A bulky and congestive mass is seen (B) edematous mucosal fold (arrow) and the lumen (arrowhead) of intussuscepted jejunum is seen.
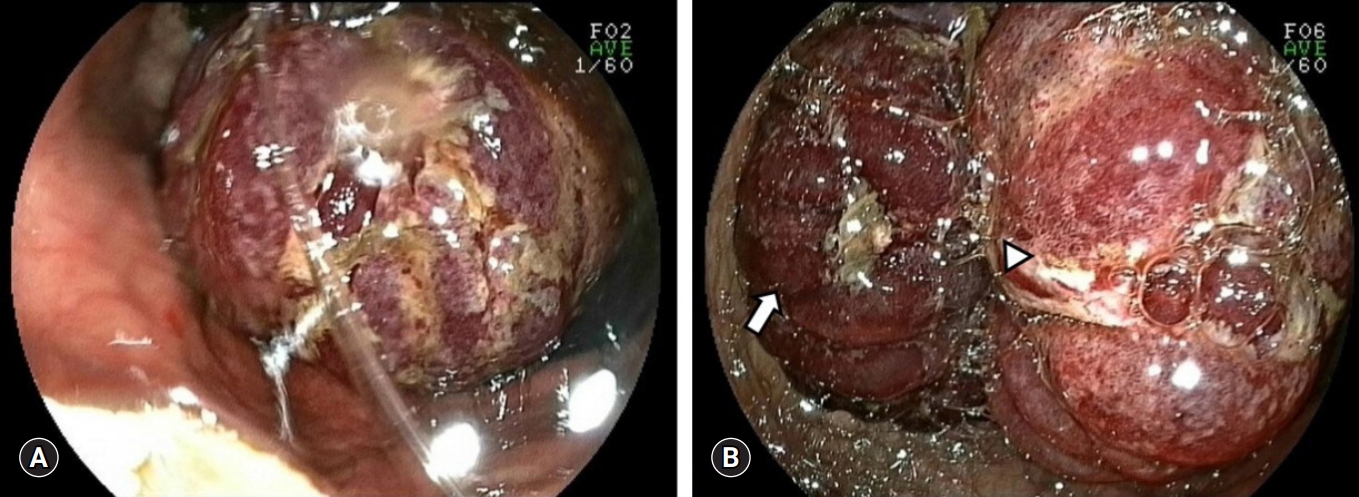

Fig. 3.Numbers of well recovered (A) and died (B) jejunogastric intussusception cases are demonstrated by published year.
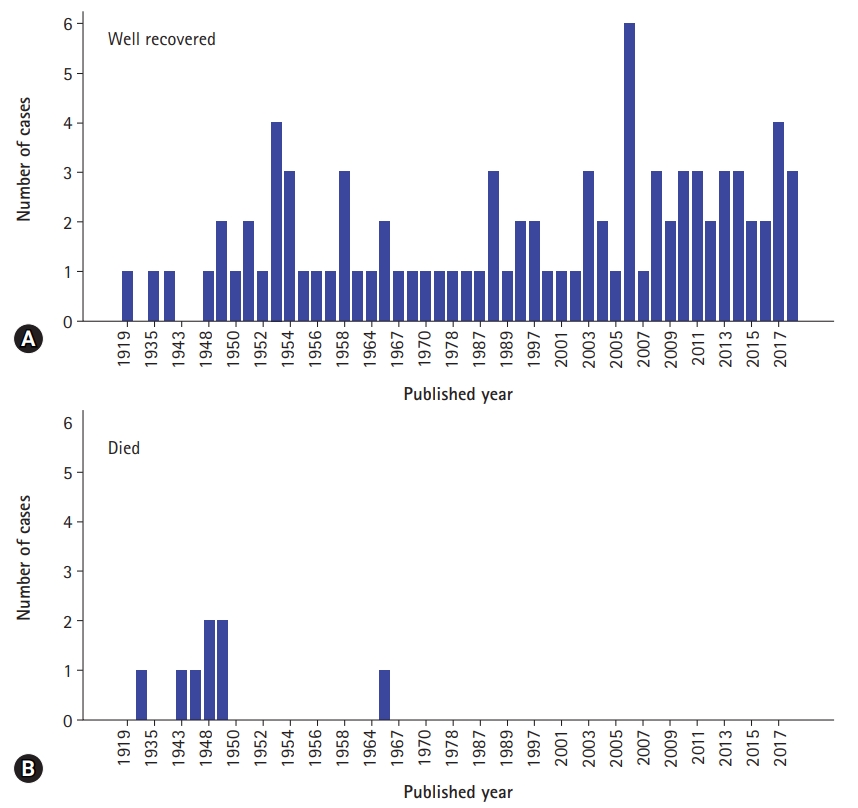

Table 1.Clinical features of jejunogastric intussusception patients
Table 2.Diagnosis, treatment, and outcomes of jejunogastric intussusception patients
| Variable | No. (%) |
|---|---|
| Upper gastrointestinal series (n=116) | |
| Not done | 53 (67.1) |
| Done | 26 (32.9) |
| N/M | 37 |
| Ultrasonography (n=116) | |
| Not done | 67 (84.8) |
| Done | 12 (15.2) |
| N/M | 37 |
| Endoscopy (n=116) | |
| Not done | 33 (41.8) |
| Done | 46 (58.2) |
| N/M | 37 |
| Computed tomography (n=116) | |
| Not done | 43 (54.4) |
| Done | 36 (45.6) |
| N/M | 37 |
| Treatment method (n=116) | |
| Spontaneous reduction | 3 (3.1) |
| Surgical reduction | 80 (83.3) |
| Endoscopic reduction | 6 (6.3) |
| Surgery after endoscopy | 2 (2.1) |
| No reduction | 5 (5.2) |
| N/M | 20 |
| Bowel resection (n=82)a) | |
| Not done | 37 (47.4) |
| Done | 41 (52.6) |
| N/M | 4 |
| Fixation (n=82)a) | |
| Not done | 46 (67.6) |
| Done | 22 (32.4) |
| N/M | 14 |
| Recurrence (n=108)b) | |
| Negative | 80 (90.9) |
| Positive | 8 (9.1) |
| N/M | 20 |
| Prognosis (n=116) | |
| Well recovered | 88 (91.7) |
| Died | 8 (8.3) |
| N/M | 20 |
- 1. Lee SH, Kwon IG, Ryu SW, Sohn SS. Jejunogastric intussusception: a rare complication of gastric cancer surgery. Int J Clin Exp Med 2014;7:4498–502.PubMedPMC
- 2. WISOFF CP. Jejunogastric intussusception. Radiology 1953;61:363–7.ArticlePubMed
- 3. Kawano F, Tashiro K, Nakao H, Fujii Y, Ikeda T, Takeno S, et al. Jejunogastric intussusception after distal gastrectomy with Roux-en-Y reconstruction: a case report. Int J Surg Case Rep 2018;44:105–9.ArticlePubMedPMC
- 4. Tokue H, Tsushima Y, Arai Y, Endo K. Jejunogastric intussusception: life-threatening complication occuring 55 years after gastrojejunostomy. Intern Med 2009;48:1657–60.ArticlePubMed
- 5. Shackman R. Jejunogastric intussusception. Br J Surg 1940;27:475–80.Article
- 6. Brynitz S, Rubinstein E. Hematemesis caused by jejunogastric intussusception. Endoscopy 1986;18:162–4.ArticlePubMedPDF
- 7. Kitasato Y, Midorikawa R, Uchino Y, Saku S, Minami T, Shirahama T, et al. A case of retrograde intussusception at Roux-en-Y anastomosis 10 years after total gastrectomy: review of the literature. Surg Case Rep 2016;2:123.ArticlePubMedPMCPDF
- 8. Hocking MP, McCoy DM, Vogel SB, Kaude JV, Sninsky CA. Antiperistaltic and isoperistaltic intussusception associated with abnormal motility after Roux-en-Y gastric bypass: a case report. Surgery 1991;110:109–12.PubMed
- 9. Bundrick TJ, Turner MA. Retrograde jejunogastric intussusception. Rev Interam Radiol 1981;6:21–4.PubMed
- 10. Sachdev BS, Malhotra P, Sukanya B, Prasad L, Kapoor D. Post gastro-jejunostomy acute retrograde jejuno-gastric intussusception. Trop Gastroenterol 2010;31:329–32.PubMed
- 11. Reyelt WP Jr, Anderson AA. retrograde jejunogastric intussusception. Surg Gynecol Obstet 1964;119:1305–11.PubMed
- 12. Czerniak A, Bass A, Bat L, Shemesh E, Avigad I, Wolfstein I. Jejunogastric intussusception. A new diagnostic test. Arch Surg 1987;122:1190–2.ArticlePubMed
- 13. Hashimoto Y, Akagi S, Sakashita Y, Takamura M, Iwako H, Watadani Y, et al. Usefulness of computed tomography as a preoperative diagnostic modality in a case with acute jejunogastric intussusception. J Gastrointest Surg 2007;11:1078–80.ArticlePubMedPDF
- 14. Singh S, Singh A, Bhagat S, Singh B. Retrograde Jejuno-gastric Intussusception. Niger J Surg 2015;21:70–2.ArticlePubMedPMC
- 15. Rather SA, Dar TI, Wani RA, Khan A. Jejunogastric intussusception presenting as tumor bleed. J Emerg Trauma Shock 2010;3:406–8.ArticlePubMedPMC
- 16. Toth E, Arvidsson S, Thorlacius H. Endoscopic reduction of a jejunogastric intussusception. Endoscopy 2011;43(Suppl 2 UCTN):E63.ArticlePubMedPDF
- 17. Guadagni S, Pistoia M, Catarci M, Carboni F, Lombardi L, Carboni M. Retrograde jejunogastric intussusception: is endoscopic or surgical management more appropriate? Surg Today 1992;22:269–72.ArticlePubMedPDF
- 18. Walstad PM, Ritter JA, Arroz V. Delayed jejunogastric intussusception after gastric surgery: an ever-present threat. Am Surg 1972;38:172–5.PubMed
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Jejunogastric intussusception after pancreaticoduodenectomy: a case report
Konosuke Yogo, Masanori Sando, Ryutaro Kobayashi, Genta Yano, Noriaki Ohara, Kiyotaka Kawai, Kenji Takagi, Satoru Kawai, Satoaki Kamiya
Surgical Case Reports.2022;[Epub] CrossRef

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite




