Indexed in: ESCI, Scopus, PubMed,
PubMed Central, CAS, DOAJ, KCI
PubMed Central, CAS, DOAJ, KCI
FREE article processing charge

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 38(2); 2021 > Article
-
Case report
High-grade mucoepidermoid carcinoma in the thyroid gland with poor prognosis -
Hyeong Chan Shin

-
Yeungnam University Journal of Medicine 2021;38(2):169-174.
DOI: https://doi.org/10.12701/yujm.2021.00941
Published online: March 5, 2021
Department of Pathology, Keimyung University School of Medicine, Daegu, Korea
- Corresponding author: Hyeong Chan Shin, MD Department of Pathology, Keimyung University School of Medicine, 1095 Dalgubeol-daero, Dalseo-gu, Daegu 42601, Korea Tel: +82-53-258-4264 Fax: +82-53-258-7382 E-mail: chan@dsmc.or.kr
• Received: January 25, 2021 • Revised: February 4, 2021 • Accepted: February 8, 2021
Copyright © 2021 Yeungnam University College of Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 5,552 Views
- 91 Download
- 4 Crossref
Abstract
- Mucoepidermoid carcinoma (MEC) is the most common malignant neoplasm of the salivary gland, but primary thyroid MEC has rarely been reported and usually has a good prognosis. Herein, I report a case of thyroidal MEC with a poor prognosis in an 82-year-old woman with an anterior neck mass. Ultrasonography and computed tomography revealed a thyroid mass. The patient initially underwent fine-needle aspiration, was diagnosed with malignancy, and underwent a right lobectomy. On gross examination, a 4.0×3.6×2.6 cm-sized ill-defined, unencapsulated, and infiltrative tan to whitish mass with necrosis was identified. Microscopically, epidermoid tumor cell nests or solid sheets were identified. Mucous cells that were positive for periodic acid–Schiff and mucicarmine stains were also identified within epidermoid cell nests. Frequent mitosis and necrosis were observed. Immunohistochemical staining for p40 and p63 was positive, and that for thyroid transcription factor-1 and paired box gene 8 was focally positive. According to the Armed Forces Institute of Pathology grading system for salivary gland MEC, the current case was classified as high-grade MEC. After surgery, the patient suffered from dyspnea due to a remnant neck mass that compressed and obstructed the trachea; therefore, the patient refused further treatment. Thyroidal MECs are considered low-grade with a favorable prognosis, but there are several reported cases of thyroidal MEC with poor prognosis. The current case is a rare presentation of high-grade thyroidal MEC with a poor prognosis.
- Mucoepidermoid carcinomas (MECs) are malignant neoplasms that mostly arise in the salivary gland [1], and also in the digestive tract, respiratory tract, pancreas, and breast [2]. In rare cases, MECs arise in the thyroid gland. MECs are classified as low-, intermediate-, or high-grade according to their histologic features [3], with most cases being classified as low-grade. However, only 49 MEC cases in the thyroid have been reported in the English literature, with nine cases having a poor prognosis [4].
- Herein, I present the case of a high-grade MEC in the thyroid of an 82-year-old woman with a poor prognosis.
Introduction
- This study was approved by the Institutional Review Board of Keimyung University Dongsan Hospital (IRB No: DSMC 2021-01-041), and the need for informed consent was waived.
- An 82-year-old woman visited our hospital because of a right anterior neck mass that persisted for a month. She had undergone a left lobectomy of the thyroid gland approximately 30 years ago at an outside hospital because of unknown causes. On ultrasonography, a 4.5 cm hypoechoic mass was identified in the right thyroid gland. On computed tomography, the mass showed as a hypodense lesion with peripheral enhancement (Fig. 1).
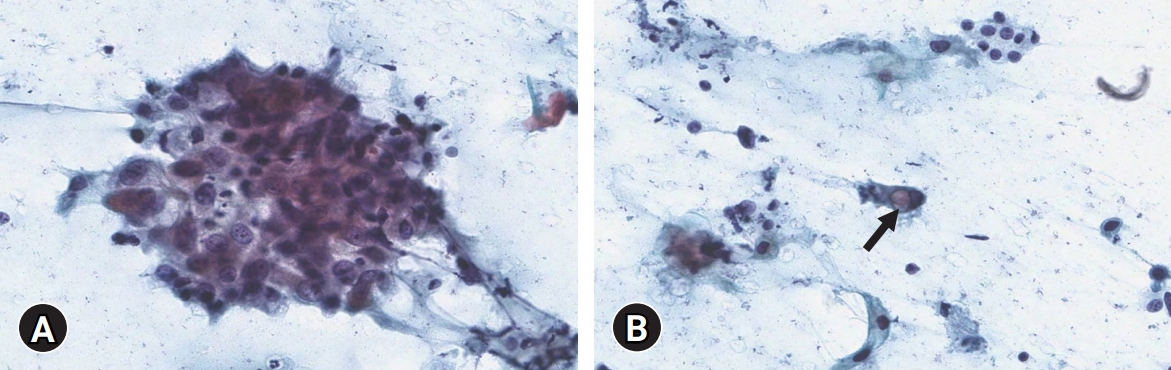
- The patient initially underwent fine-needle aspiration of the right thyroid mass. The smear showed atypical cell clusters and scattered single cells with a dirty inflammatory background. The atypical cells were polygonal-shaped. The nuclear-cytoplasmic ratio was high, and prominent nucleoli were observed. Some scattered single cells with intracytoplasmic vacuoles were identified as signet ring cells (Fig. 2). These cytologic features strongly implied malignancy.
- Two weeks later, the patient underwent right lobectomy with selective neck dissection for right level IV lymph nodes. In the surgical field, the right thyroid mass was adhered to the surrounding tissue and invaded the trachea; hence, the mass could not be completely removed.
- On gross examination, a 4.0×3.6×2.6 cm-sized ill-defined, unencapsulated, and infiltrative tan to whitish mass was identified. Necrosis was also observed in the mass (Fig. 3). The remaining thyroid gland parenchyma was unremarkable. All of the right thyroid mass was made into formalin-fixed, paraffin-embedded blocks.
- Microscopically, the right thyroid mass was composed of large irregular nests or solid sheets of tumor cells surrounded by fibrotic stroma with necrosis. The tumor cell populations consisted predominantly of polygonal epidermoid cells with abundant amphophilic cytoplasm and large nuclei having prominent nucleoli. Keratin pearls were also identified in epidermoid cell nests. Mitoses were frequently observed as up to 25 per 10 high-power fields (HPFs). Individually vacuolated cells were present within epidermoid cell nests. Special stains, periodic acid–Schiff and mucicarmine, demonstrated mucin globules in vacuolated cells (Fig. 4). There was no evidence of well-differentiated thyroid carcinomas, such as papillary thyroid carcinoma, follicular thyroid carcinoma, or medullary thyroid carcinoma. In addition, there were no anaplastic features on any of the slides.
- Immunohistochemical staining for p40, p63, thyroid transcription factor-1 (TTF-1), and paired box gene 8 (PAX8) was performed. The epidermoid cells were positive for p40 and p63 and were focally positive for TTF-1 and PAX8 (Fig. 5). These histologic findings and immunohistochemical staining results were consistent with the diagnosis of thyroidal MEC. Metastatic carcinoma with perinodal extension was also revealed in one out of seven right level IV lymph nodes.
- Radiation therapy for residual tumors was planned as a further treatment for the patient. However, 1 month after surgery, the patient visited our emergency room for dyspnea due to a cystic right neck mass that compressed and obstructed the trachea. The patient refused further adjuvant therapy and was transferred to an outside hospital.
Case
- MEC is a malignant epithelial neoplasm composed of epidermoid, mucous, intermediate, columnar, and clear cells that often demonstrate prominent cystic growth [5]. MECs are most commonly encountered as malignant neoplasms of salivary glands [6]. MECs also arise in the bronchus, breast, esophagus, and pancreas [7], and are thought to originate from intermediate cells that differentiate into epidermoid, mucous, and clear cells [5]. On immunohistochemical staining, epidermoid cells are positive for p63, p40, and cytokeratin 5/6. Therefore, these are useful markers for MECs [8].
- MECs in salivary glands are graded as low-, intermediate-, and high-grade based on morphological and cytological features. Prominent cystic components are the hallmark of low-grade MEC, and mucous cells are more frequently identified in low-grade MECs than in intermediate- and high-grade MECs [5]. In the Armed Forces Institute of Pathology (AFIP) grading system, high-grade features include a low proportion (less than 20%) of intracystic components, neural invasion, necrosis, mitotic figures, and anaplasia. A quantitative AFIP grading system was used based on the scores for each of the five histopathological features. Tumors with a score of 0 to 4 were considered low-grade, a score of 5 or 6 was intermediate-grade, and a score of 7 to 14 was considered high-grade [3]. The prognosis of high-grade MECs is very poor compared to that of intermediate- and low-grade MECs. The overall survival and disease-free survival of high-grade MECs are approximately 50%, compared to approximately 90% of intermediate- and low-grade MECs [9].
- In the thyroid, MECs are rare malignant neoplasms, accounting for 0.5% of thyroid malignancies, with approximately 49 cases reported in the English literature [4]. Patients are aged 10 to 91 years and predominantly female [1]. The origin of MECs in the thyroid gland is unclear; however, thyroid follicular epithelium [10] or solid cell nests [11] are regarded as suspicious candidates. Some authors have proposed that MECs can arise from metaplastic dedifferentiation of papillary thyroid carcinoma, follicular thyroid carcinoma, or oncocytic carcinoma [7]. The microscopic findings of MEC in the thyroid gland are similar to those in the salivary gland. Intermingled epidermoid and mucous cells are arranged in cords, nests, or solid sheets in the fibrotic stroma. Epidermoid cells have intercellular bridges and undergo keratinization. The mucous cells have abundant foamy to clear or vacuolated cytoplasm and exhibit a peripherally displaced nucleus. The foamy to clear cytoplasm is positive for periodic acid–Schiff and mucicarmine stains [12]. Mitosis and necrosis are rare. Unlike the salivary gland, a grading system for thyroidal MEC has not yet been established. MECs in the thyroid are generally considered low-grade malignancies and have a favorable prognosis. However, poor prognostic cases of MECs have also been reported [4].
- In the current case, the tumor had large irregular nests or solid sheets of tumor cells surrounded by fibrotic stroma. The tumor was composed of epidermoid cells with prominent nucleoli, keratin pearls, and vacuolated mucous cells. Necrosis and frequent mitoses were also identified. Based on the AFIP grading system [3], the score of the current case was eight (Table 1) and was therefore classified as high-grade. The patient’s hospital course matched the tumor grade. As previously described, MECs in the thyroid may be associated with well-differentiated thyroid tumors [7]; however, in the current case, there was no evidence of a well-differentiated thyroid tumor on any of the slides.
- The differential diagnosis of high-grade MEC includes poorly differentiated thyroid carcinoma (PDTC), anaplastic thyroid carcinoma (ATC), sclerosing MEC with eosinophilia (SMECE), and squamous cell carcinoma (SCC). PDTC is a rare follicular cell-derived thyroid tumor with limited evidence of thyroid follicular differentiation. Histologically, PDTC is a solid, trabecular, and insular growth pattern with convoluted nuclei, increased mitotic activity (three or more mitoses per 10 HPFs), and coagulative tumor necrosis. No mucous cells are observed in PDTC. Immunohistochemically, PDTC is positive for TTF-1 and PAX8 but negative for p63 and p40 [13]. ATC is a highly aggressive thyroid malignancy that is composed of undifferentiated thyroid follicular cells. Histologically, ATC is composed of sarcomatoid spindle cells and giant cells that are morphologically indistinguishable from sarcomas. Immunohistochemically, ATC is positive for PAX8 but negative for TTF-1, p63, and p40 [14]. SMECE is a rare thyroid tumor derived from the metaplastic squamous epithelium or solid cell nests. The histologic findings of SMECE include squamous and mucus-secreting cells within a fibrohyaline stroma and eosinophilic infiltrates in the background of Hashimoto thyroiditis [15]. SCC of the thyroid gland is an extremely rare thyroid tumor composed entirely of tumor cells with squamous differentiation without mucous cells [16].
- Initially, fine-needle aspiration cytology revealed polygonal-shaped malignant squamoid cells. The differential diagnosis of the current case included MEC, ATC, diffuse sclerosing variant of papillary thyroid carcinoma (DSVPTC), and carcinoma showing thymus-like differentiation (CASTLE). In ATC, the smear shows markedly anaplastic, spindle, or squamoid cells with marked nuclear pleomorphism. In DSVPTC, the smear shows cohesive epithelial cell clusters composed of squamoid cells with classic papillary thyroid carcinoma features [17]. In CASTLE, a single epithelial cell with three-dimensional fragments of cohesive epithelioid cells with squamoid and basaloid features is observed [18]. In the current case, vacuolated cells were observed. Also, classic papillary thyroid carcinoma features were not identified.
- Thyroidal MEC is a rare malignant neoplasm, with only 49 reported cases to date [4]. Commonly, this rare malignant tumor is considered low-grade, but several cases with poor outcomes have also been reported [1,4]. Herein, a very rare case of primary thyroid high-grade MEC with a poor prognosis has been described.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Notes
Fig. 1.Radiological findings. (A) A 4.5 cm-sized hypoechoic mass (arrow) on ultrasonography and (B) a hypoechoic mass (arrow) with peripheral enhancement on computed tomography.
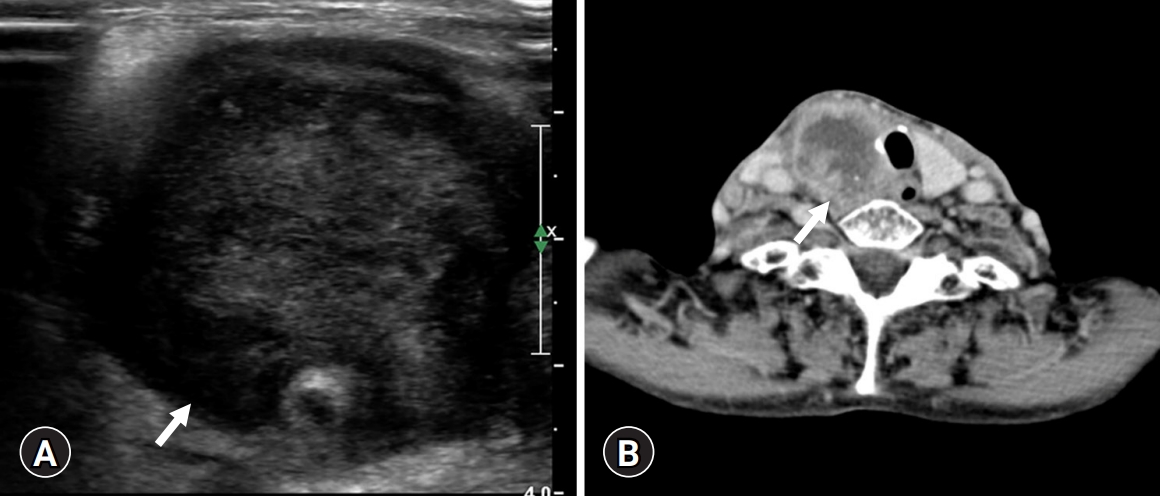

Fig. 2.Cytological findings. (A) Polygonal-shaped atypical cell clusters with high nuclear-cytoplasmic ratio and prominent nucleoli are seen (Papanicolaou stain, ×400). (B) Scattered single cells with intracytoplasmic vacuole (arrow) are identified (Papanicolaou stain, ×400).
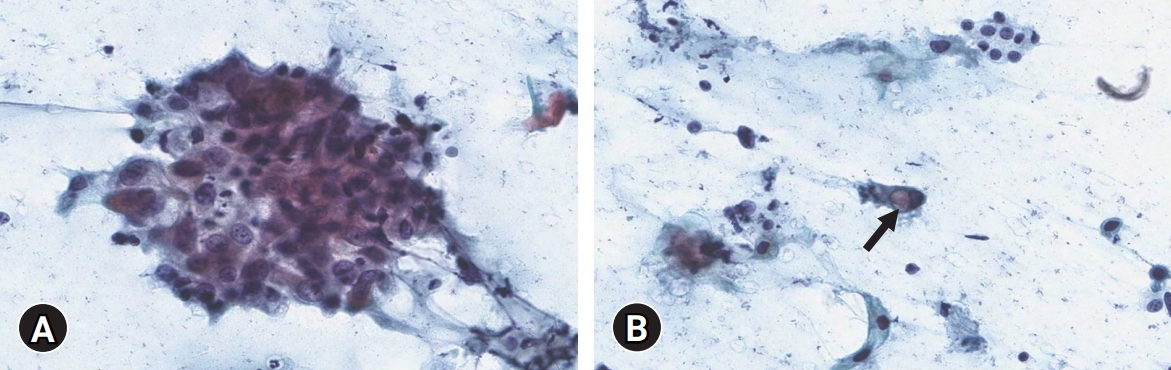

Fig. 3.Gross findings. A 4.0×3.6×2.6 cm-sized ill-defined infiltrative tan to whitish mass with necrosis is present.
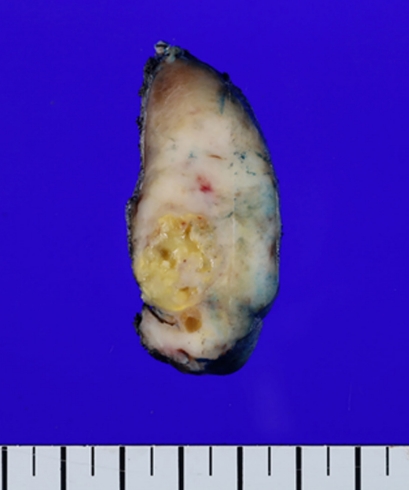

Fig. 4.Histological findings. (A) Large irregular nests or solid sheets of tumor cells surrounded by fibrotic stroma with necrosis are identified (hematoxylin and eosin [H&E] stain, ×100). (B) The epidermoid cells have prominent nucleoli and keratin pearl formation (H&E stain, ×200). (C) Mitoses are frequently identified (H&E stain, ×400). (D) Vacuolated cells are located within epidermoid cells (H&E stain, ×400). (E) Mucicarmine stain highlights intracytoplasmic mucin droplets (mucicarmine stain, ×400).
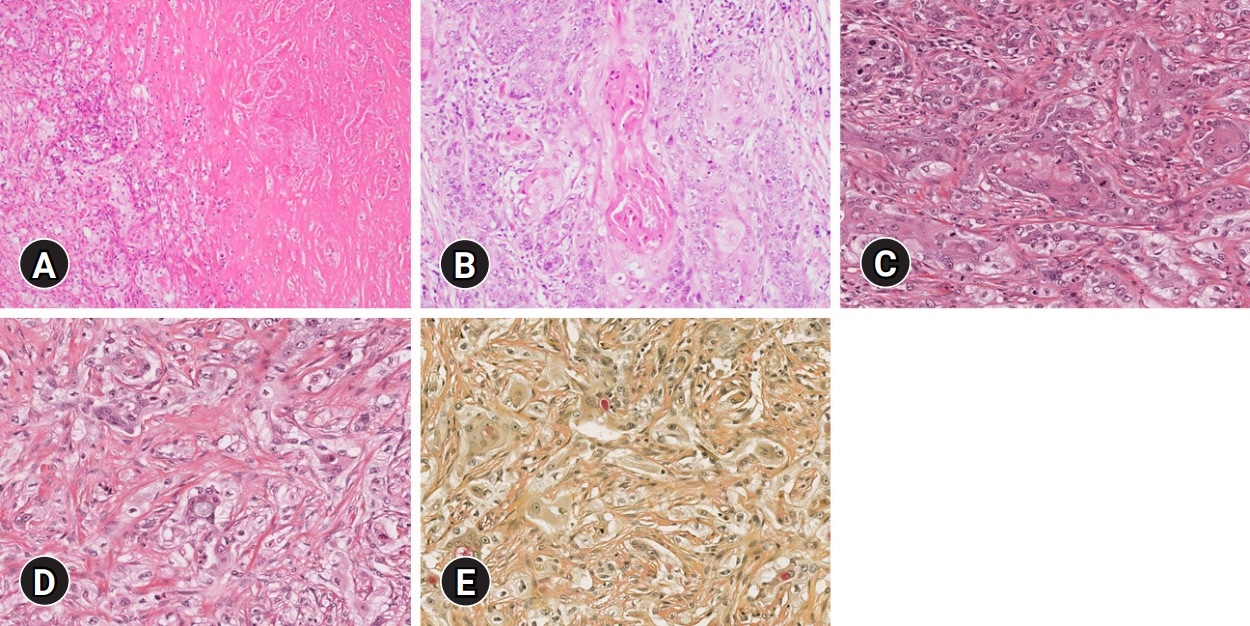

Fig. 5.Immunohistochemical results. The epidermoid cells are diffusely positive for (A) p63 and focally positive for (B) thyroid transcription factor-1 and (C) paired box gene 8 (immunohistochemical stain, x400).
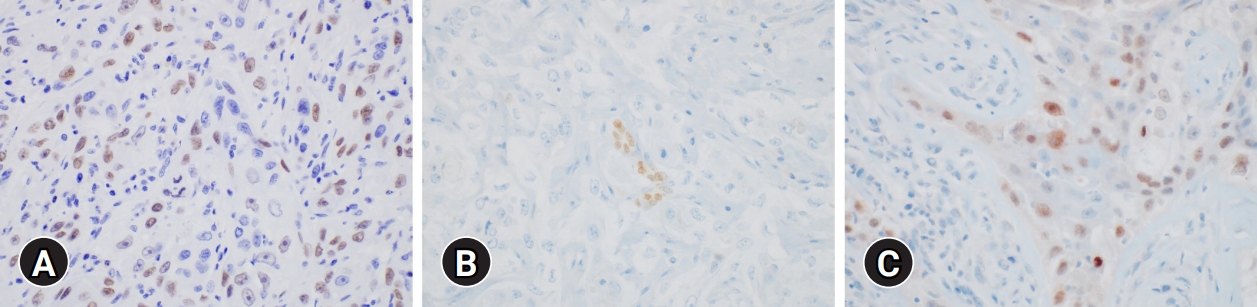

Table 1.The point score and grade of present case according to Armed Forces Institute of Pathology (AFIP) grading system for mucoepidermoid carcinoma
- 1. Le QV, Ngo DQ, Ngo QX. Primary mucoepidermoid carcinoma of the thyroid: a report of a rare case with bone metastasis and review of the literature. Case Rep Oncol 2019;12:248–59.ArticlePubMedPMC
- 2. Bhandarkar ND, Chan J, Strome M. A rare case of mucoepidermoid carcinoma of the thyroid. Am J Otolaryngol 2005;26:138–41.ArticlePubMed
- 3. Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer 1998;82:1217–24.ArticlePubMed
- 4. Lee K, Mirza O, Dobbs S, Jayaram S. Poorly differentiated mucoepidermoid carcinoma of the thyroid. BMJ Case Rep 2020;13:e236539.ArticlePubMedPMC
- 5. Luna MA. Salivary mucoepidermoid carcinoma: revisited. Adv Anat Pathol 2006;13:293–307.ArticlePubMed
- 6. Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol 2001;25:835–45.ArticlePubMed
- 7. Prichard RS, Lee JC, Gill AJ, Sywak MS, Fingleton L, Robinson BG, et al. Mucoepidermoid carcinoma of the thyroid: a report of three cases and postulated histogenesis. Thyroid 2012;22:205–9.ArticlePubMed
- 8. Butler RT, Spector ME, Thomas D, McDaniel AS, McHugh JB. An immunohistochemical panel for reliable differentiation of salivary duct carcinoma and mucoepidermoid carcinoma. Head Neck Pathol 2014;8:133–40.ArticlePubMed
- 9. McHugh CH, Roberts DB, El-Naggar AK, Hanna EY, Garden AS, Kies MS, et al. Prognostic factors in mucoepidermoid carcinoma of the salivary glands. Cancer 2012;118:3928–36.ArticlePubMed
- 10. Minagawa A, Iitaka M, Suzuki M, Yasuda S, Kameyama K, Shimada S, et al. A case of primary mucoepidermoid carcinoma of the thyroid: molecular evidence of its origin. Clin Endocrinol (Oxf) 2002;57:551–6.ArticlePubMed
- 11. Ando M, Nakanishi Y, Asai M, Maeshima A, Matsuno Y. Mucoepidermoid carcinoma of the thyroid gland showing marked ciliation suggestive of its pathogenesis. Pathol Int 2008;58:741–4.ArticlePubMed
- 12. Vázquez Ramírez F, Otal Salaverri C, Argueta Manzano O, Galera Ruíz H, González-Cámpora R. Fine needle aspiration cytology of high grade mucoepidermoid carcinoma of the thyroid: a case report. Acta Cytol 2000;44:259–64.ArticlePubMed
- 13. Setia N, Barletta JA. Poorly differentiated thyroid carcinoma. Surg Pathol Clin 2014;7:475–89.ArticlePubMed
- 14. Ragazzi M, Ciarrocchi A, Sancisi V, Gandolfi G, Bisagni A, Piana S. Update on anaplastic thyroid carcinoma: morphological, molecular, and genetic features of the most aggressive thyroid cancer. Int J Endocrinol 2014;2014:790834.ArticlePubMedPMC
- 15. Shah AA, La Fortune K, Miller C, Mills SE, Baloch Z, LiVolsi V, et al. Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia: a clinicopathologic and molecular analysis of a distinct entity. Mod Pathol 2017;30:329–39.ArticlePubMed
- 16. Suzuki A, Hirokawa M, Takada N, Higuchi M, Yamao N, Kuma S, et al. Diagnostic significance of PAX8 in thyroid squamous cell carcinoma. Endocr J 2015;62:991–5.ArticlePubMed
- 17. Rossi ED, Faquin WC, Pantanowitz L. Cytologic features of aggressive variants of follicular-derived thyroid carcinoma. Cancer Cytopathol 2019;127:432–46.ArticlePubMedPMC
- 18. Collins JA, Ping B, Bishop JA, Ali SZ. Carcinoma showing thymus-like differentiation (CASTLE): cytopathological features and differential diagnosis. Acta Cytol 2016;60:421–8.ArticlePubMed
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Primary mucoepidermoid carcinoma of the thyroid with concomitant MAML2 gene rearrangement and BRAF V600E mutation – A case report
Frederica Loghides, Brigid Aherne
Oral Oncology Reports.2024; 9: 100154. CrossRef - Mucoepidermoid carcinoma of the pancreas: A case report and literature review
Huan Zhang, Shuyan Wang, Chunnian Wang
Medicine.2024; 103(4): e36993. CrossRef - Primary Thyroid Mucoepidermoid Carcinoma (MEC) Is Clinically, Prognostically, and Molecularly Different from Sclerosing MEC with Eosinophilia: A Multicenter and Integrated Study
Hieu Trong Le, Truong P. X. Nguyen, Mitsuyoshi Hirokawa, Ryohei Katoh, Norisato Mitsutake, Michiko Matsuse, Ayaka Sako, Tetsuo Kondo, Nilesh Vasan, Young Mi Kim, Ying Liu, Lewis Hassell, Kennichi Kakudo, Huy Gia Vuong
Endocrine Pathology.2023; 34(1): 100. CrossRef - Overview of the 2022 WHO Classification of Thyroid Neoplasms
Zubair W. Baloch, Sylvia L. Asa, Justine A. Barletta, Ronald A. Ghossein, C. Christofer Juhlin, Chan Kwon Jung, Virginia A. LiVolsi, Mauro G. Papotti, Manuel Sobrinho-Simões, Giovanni Tallini, Ozgur Mete
Endocrine Pathology.2022; 33(1): 27. CrossRef

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite