PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(Suppl); 2023 > Article
-
Original article
Diagnostic performance of F-18 FDG PET or PET/CT for detection of recurrent gastric cancer: a systematic review and meta-analysis -
Chang In Choi
 , Jae Kyun Park
, Jae Kyun Park , Tae Yong Jeon
, Tae Yong Jeon , Dae-Hwan Kim
, Dae-Hwan Kim
-
Journal of Yeungnam Medical Science 2023;40(Suppl):S37-S46.
DOI: https://doi.org/10.12701/jyms.2023.00220
Published online: August 17, 2023
Department of Surgery and Biomedical Research Institute, Pusan National University Hospital, Pusan National University College of Medicine, Busan, Korea
- Corresponding author: Dae-Hwan Kim, MD, PhD Department of Surgery and Biomedical Research Institute, Pusan National University Hospital, Pusan National University College of Medicine, 179 Gudeok-ro, Seo-gu, Busan 49241, Korea Tel: +82-51-240-7238 • Fax: +82-51-247-1365 • E-mail: dh2-kim@pusan.ac.kr
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,419 Views
- 51 Download
Abstract
-
Background
- This systematic review and meta-analysis investigated the diagnostic performance of F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) or PET/computed tomography (PET/CT) for the detection of disease recurrence after curative resection of gastric cancer.
-
Methods
- The PubMed and Embase databases, from the earliest available date of indexing through November 30, 2019, were searched for studies evaluating the diagnostic performance of F-18 FDG PET or PET/CT to detect recurrent disease after gastric cancer surgery.
-
Results
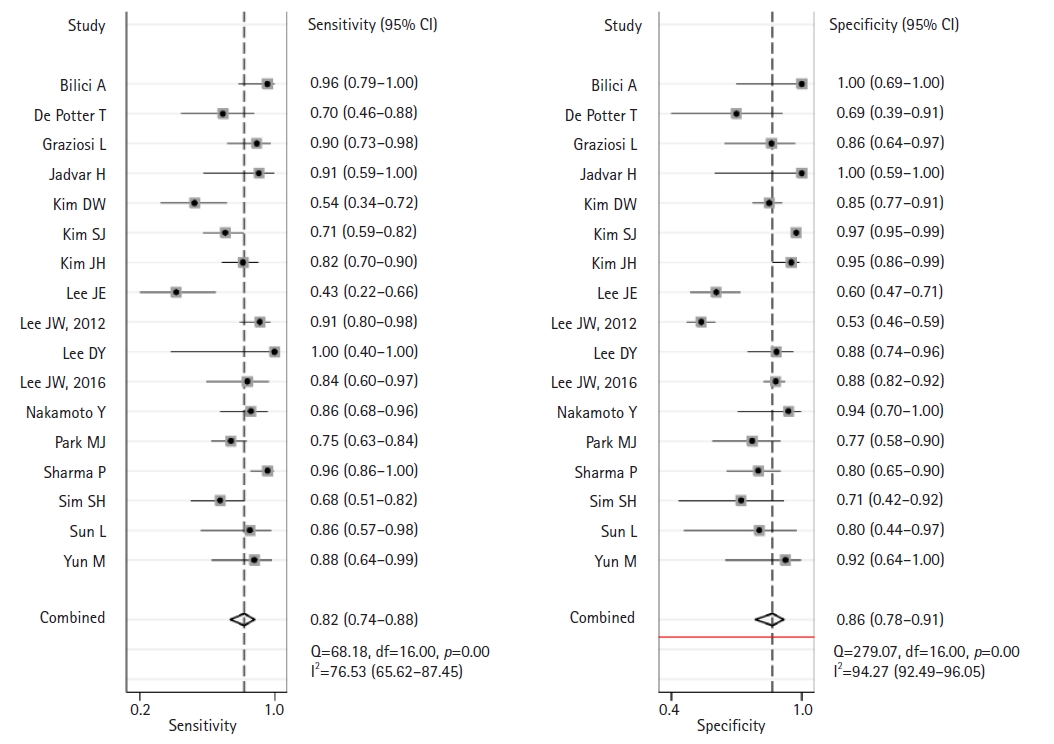
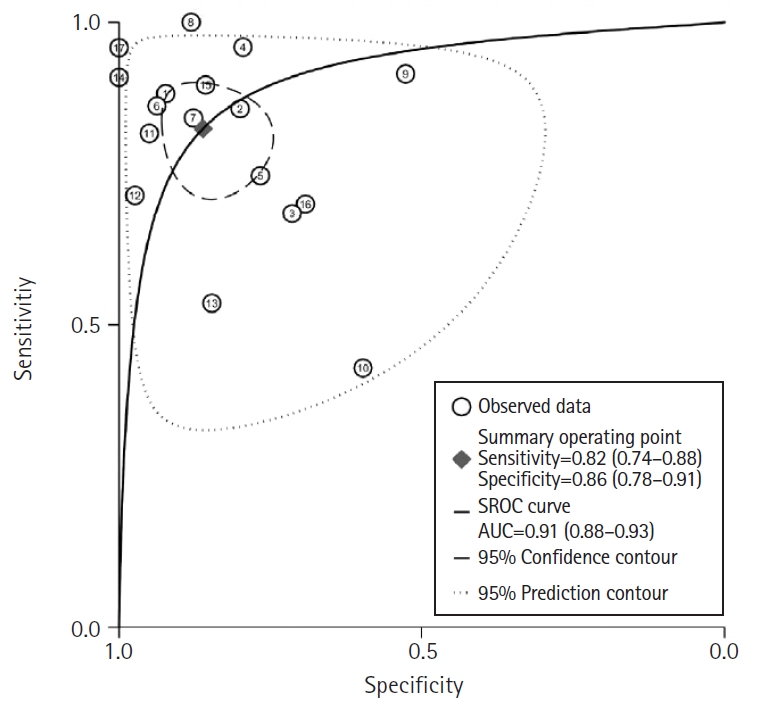
- Across 17 studies (1,732 patients), the pooled sensitivity for F-18 FDG PET or PET/CT was 0.82 (95% confidence interval [CI], 0.74–0.88) with heterogeneity of I2=76.5 (p<0.001), and the specificity was 0.86 (95% CI, 0.78–0.91) with heterogeneity of I2=94.2 (p<0.001). Likelihood ratio (LR) tests gave an overall positive LR of 6.0 (95% CI, 3.6–9.7) and negative LR of 0.2 (95% CI, 0.14–0.31). The pooled diagnostic odds ratio was 29 (95% CI, 13–63). The summary receiver operating characteristic curve indicates that the area under the curve was 0.91 (95% CI, 0.88–0.93).
-
Conclusion
- The current meta-analysis showed good sensitivity and specificity of F-18 FDG PET or PET/CT for detecting recurrent disease after curative resection of gastric cancer despite heterogeneity in ethnicity, recurrence rate, histology, and interpretation method.
- Gastric cancer is the fifth most common cancer worldwide, and its incidence has decreased [1]. According to the National Cancer Center Registry, in 2020, gastric cancer was the fourth most common cancer (10.8%) in Korea after thyroid (11.8%), lung (11.7%), and colorectal (11.2%) cancers. However, owing to its high prevalence and mortality, gastric cancer remains an important health issue [2].
- Complete resection with regional lymph node dissection is the treatment of choice for resectable cancers. In some cases, additional adjuvant chemotherapy is required to prevent recurrence [3]. Although the survival rate of gastric cancer has increased, careful surveillance is an essential part of the postoperative management of patients during the follow-up period because the 5-year survival rate of patients with advanced gastric cancer (regional stage) is still poor (61.8%) [4]. The primary tools currently used to diagnose recurrence are tumor markers (carcinoembryonic antigen [CEA] and carbohydrate antigen 19-9 [CA19-9]), computed tomography (CT), and endoscopy.
- F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) and PET/CT are well-established imaging modalities for tumor staging in different types of cancer [5,6]. The diagnostic accuracy of PET/CT is high and it is considered a critical tool in cancer imaging [7]. However, despite their usefulness, F-18 FDG PET or PET/CT has limitations in detecting primary gastric cancer, particularly signet ring cell carcinoma (SRC) and mucinous adenocarcinoma (MA) lesions. Moreover, the accuracy of F-18 FDG PET or PET/CT for detecting recurrent gastric cancer after curative resection remains controversial [8,9]. Due to these limitations, PET/CT is not routinely used for surveillance. Therefore, further research is needed on the diagnostic performance of PET/CT for detecting recurrent disease after curative resection of gastric cancer.
- Our study aimed to perform a meta-analysis of published data on the diagnostic accuracy of F-18 FDG PET or PET/CT in detecting recurrent disease after curative resection of gastric cancer. This will provide evidence-based data that can guide future studies on patient diagnosis and treatment of recurrent disease.
Introduction
- 1. Data sources and search strategy
- To obtain data for our study, we conducted electronic English-language literature searches on the PubMed and Embase databases from the earliest available date of indexing through November 30, 2019. In addition, we manually searched the reference lists of the identified publications to find any relevant studies that may have been missed. Our search algorithm was based on a combination of relevant terms and keywords to ensure a comprehensive and targeted search: (1) “PET” OR “positron emission tomography” OR “positron emission tomography/computed tomography” OR “PET/CT” OR “positron emission tomography-computed tomography” OR “PET-CT” OR “FDG” OR “Fluorodeoxyglucose” and (2) “Stomach neoplasms” OR “Stomach cancer” OR “Gastric cancer” and (3) “Recurrence” OR “Restaging” OR “Relapse” OR “Metastasis.”
- 2. Study selection
- Certain inclusion criteria were used to identify relevant studies for this review. These studies used F-18 FDG PET or PET/CT to detect recurrent disease in patients who underwent curative resection for gastric cancer. Additionally, the studies had to provide sufficient data for the reassessment of the sensitivity and specificity of F-18 FDG PET or PET/CT for detecting recurrent disease or they had to provide absolute numbers of true positive (TP), true negative (TN), false positive (FP), and false negative (FN) data. Finally, studies with overlapping data, duplicate publications, and articles that did not contain original data, such as review articles, case reports, conference papers, and letters, were excluded. Two researchers independently reviewed the titles and abstracts of articles that met the criteria mentioned above. Ineligible articles were excluded. The same researchers then independently evaluated the full text of the included articles to determine whether they met the eligibility criteria for inclusion in this review.
- 3. Data extraction and quality assessment
- For this review, we collected information about the authors, year of publication, and country of origin of each study, as well as whether the study was performed prospectively or retrospectively, patient characteristics, and technical aspects. We then analyzed each study to determine the number of TP, TN, FP, and FN values for F-18 FDG PET and PET/CT when detecting recurrent disease in patients with gastric cancer who had undergone curative resection. We included only studies that provided complete information for our meta-analysis.
- To ensure the quality of the studies, two authors independently and critically appraised each study using a modified version of the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool with 15 items [10]. Any discrepancies between the two authors were resolved through discussion.
- 4. Data synthesis and analysis
- All data from the eligible studies were extracted to estimate sensitivity and specificity, positive and negative likelihood ratios (LR+ and LR–, respectively), and diagnostic odds ratios (DORs) with 95% confidence intervals (CIs). The DOR is a measure of discriminatory test performance that compares the odds of positivity in a disease state to the odds of positivity in a non-disease state, with higher values indicating better performance [11]. We assessed the between-study statistical heterogeneity using I2 and the Cochrane Q test based on random effects analysis [12]. To examine publication bias, we used an effective sample size funnel plot and an associated regression test for asymmetry as described by Deeks et al. [13]. We used the bivariate random effects model to analyze and pool diagnostic performance measures across studies and to compare different index tests [14,15]. The model estimates pairs of logit-transformed sensitivity and specificity values from the studies, incorporating any correlation that might exist between sensitivity and specificity. A summary receiver operating characteristic (SROC) curve was formed based on individual study data points to reveal the pooled accuracy [16]. When the statistical heterogeneity was substantial, we performed a meta-regression to identify potential sources of bias [17]. A two-sided p-value of ≤0.05 was considered statistically significant. All statistical analyses were performed using a commercial software program (STATA, ver. 13.1; StataCorp LP., College Station, TX, USA).
Methods
- 1. Literature search and selection of studies
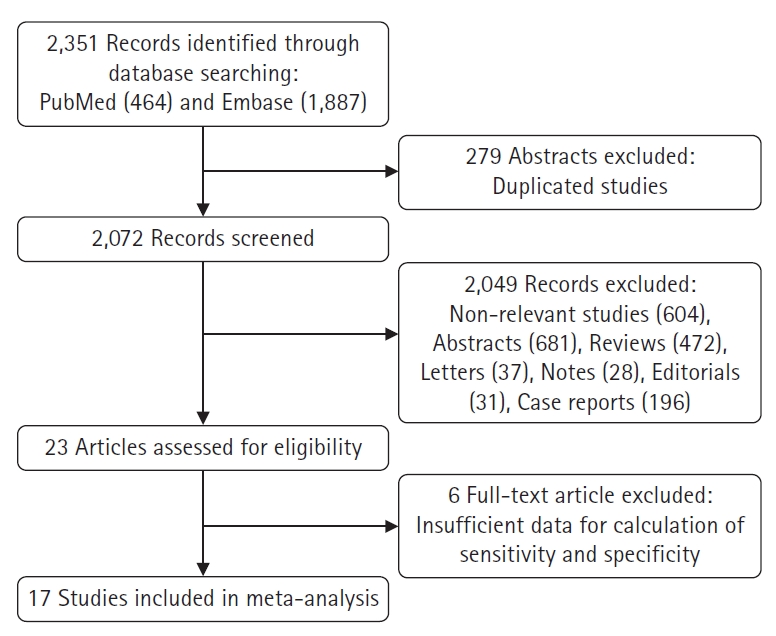
- Following a comprehensive computerized search and extensive cross-checking of the reference lists, our research yielded 2,351 records. Of these, 279 duplicate abstracts were excluded after reviewing their titles and abstracts. Additionally, 604 nonrelevant studies, 196 case reports, 681 conference abstracts, 28 notes, 37 letters, 31 editorials, and 472 review articles were excluded. After assessing the eligibility of the remaining 23 full-text articles, six were excluded because of insufficient data for the calculation of sensitivity and specificity of F-18 FDG PET or PET/CT for detecting recurrent disease after curative resection in patients with gastric cancer. Ultimately, 17 studies were selected and deemed eligible for systematic review and meta-analysis; no additional studies were found upon screening the references of these articles [8,9,18-32]. Table 1 summarizes the characteristics of the included studies. A detailed outline of the study selection procedure is shown in Fig. 1.
- 2. Study description, quality, and publication bias
- The present study analyzed the per-patient and per-lesion data. The included studies included 1,732 patients with ages ranging from 27 to 87 years. Of these, 1,109 were male, and 573 were female. The sexes of the 50 patients included in the study by Graziosi et al. [18] were not specified. All the studies were conducted retrospectively. The median prevalence of disease recurrence after curative resection was 53.3% in the 14 included studies, ranging from 8.4% to 70.6%. The median proportion of SRC and MA in the same studies was 13.3%, ranging from 5.8% to 26.9%. Three studies employed a quantitative method for interpreting F-18 FDG PET or PET/CT images, all of which used maximum standardized uptake value to detect disease recurrence after curative resection of gastric cancer [8,22,23]. The remaining 14 studies visually interpreted F-18 FDG PET or PET/CT images [10,18-20,23-32]. Of the 17 studies included in this meta-analysis, three analyzed F-18 FDG PET and were published between 2003 and 2011. Thirteen studies published between 2002 and 2017 analyzed PET/CT. One study published in 2009 analyzed both imaging types.
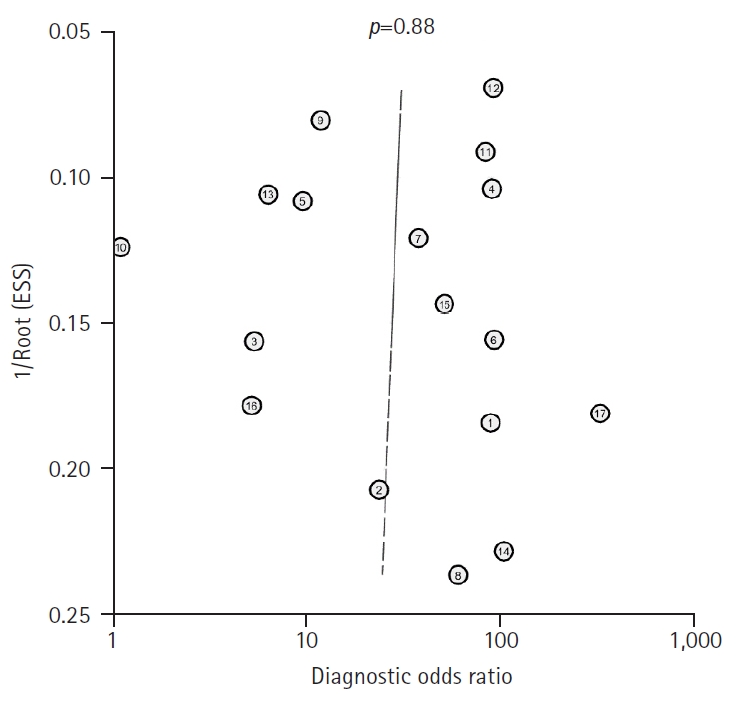
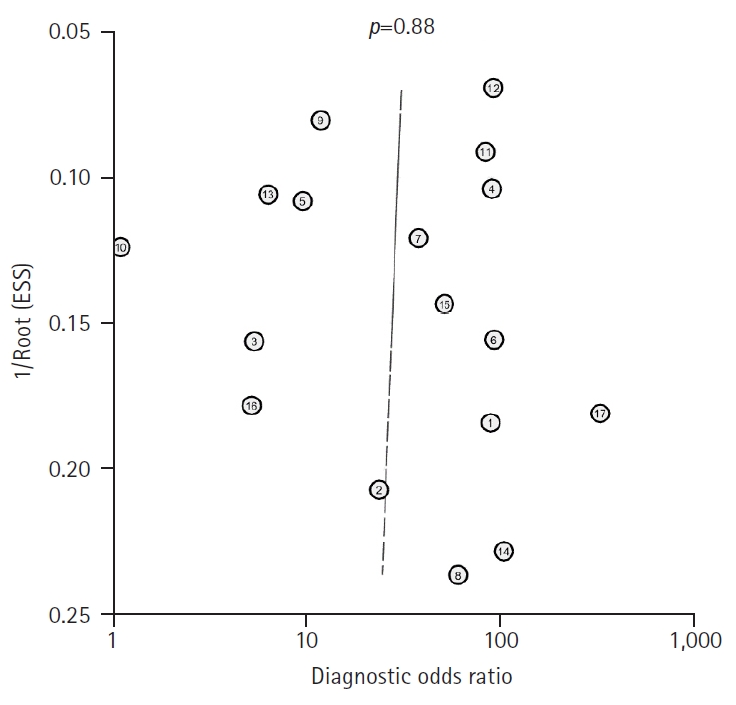
- Table 1 provides a comprehensive overview of the principal characteristics of the 17 included studies. We used Deeks’ funnel plot asymmetry tests to examine the possibility of publication bias, which revealed a non-significant slope, indicating the absence of significant bias (p=0.88) (Fig. 2).
- 3. Methodological quality assessment
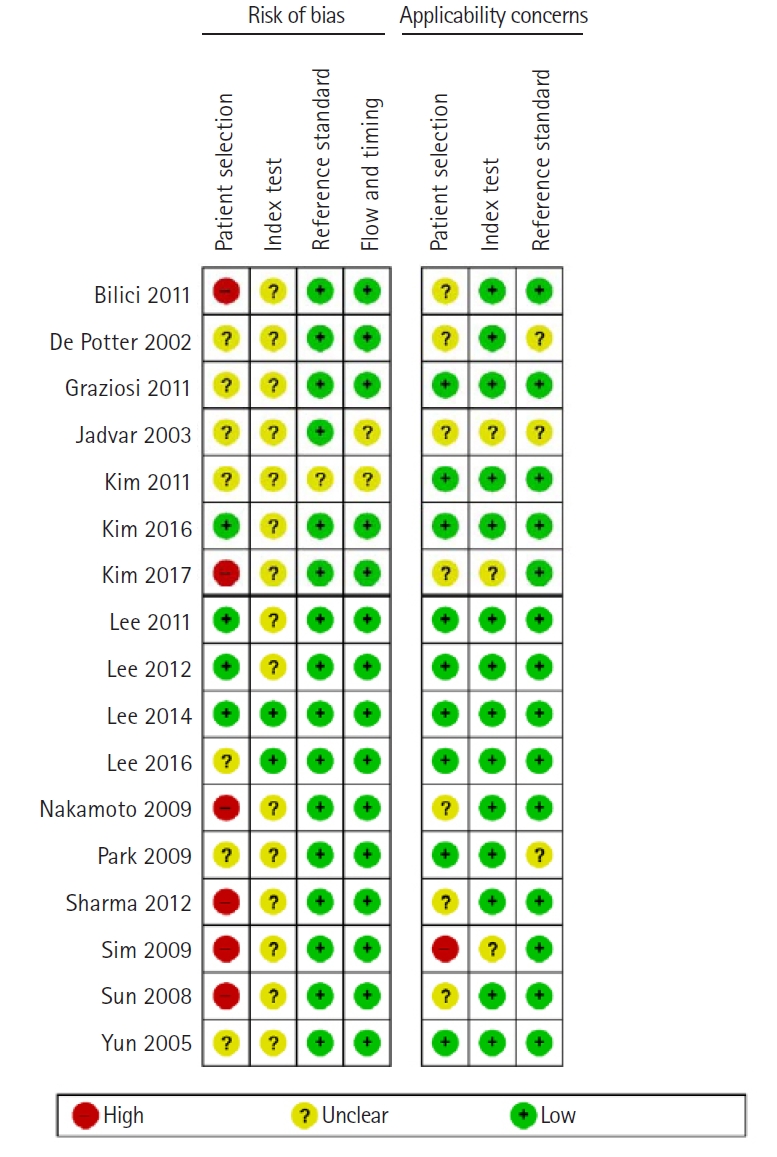
- The summary of the risk of bias and applicability concerns illustrated in Fig. 3 reveal that the quality of the studies included in this analysis was satisfactory.
- 4. Diagnostic performance of F-18 FDG PET or PET/CT
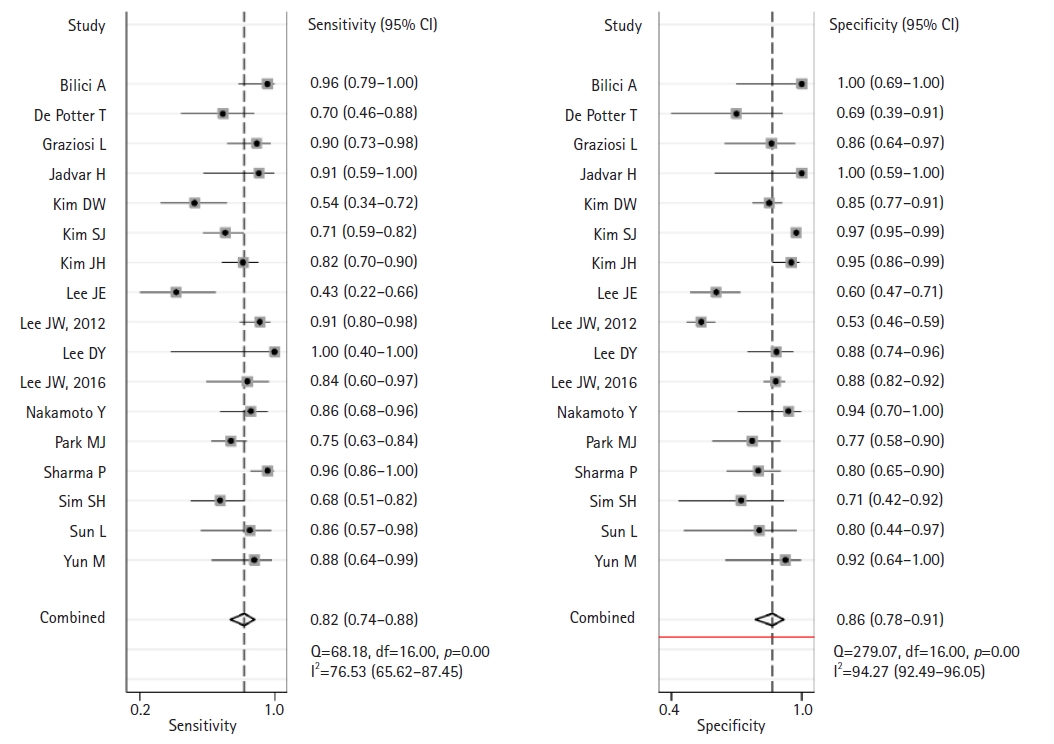
- This meta-analysis of the data from 17 studies assessed the diagnostic performance of F-18 FDG PET and PET/CT in detecting disease recurrence in patients who underwent curative resection for gastric cancer. This assessment is presented in Fig. 4.
- The pooled sensitivity of F-18 FDG PET or PET/CT was 0.82 (95% CI, 0.74–0.88) with significant heterogeneity (I2=76.5; 95% CI, 65.6–87.4; p<0.001). Similarly, the pooled specificity was 0.86 (95% CI, 0.78–0.91) with considerable heterogeneity (I2=94.2; 95% CI, 92.4–96.1; p<0.001). The likelihood ratio tests showed an overall LR+ of 6.0 (95% CI, 3.6–9.7) and LR– of 0.2 (95% CI, 0.14–0.31). The pooled DOR was 29 (95% CI, 13–63). The forest plots in Fig. 4 depict the sensitivity and specificity of F-18 FDG PET or PET/CT for detecting recurrent disease following curative resection of gastric cancer. The SROC curve in Fig. 5 shows an area under the curve of 0.91 (95% CI, 0.88–0.93).
- 5. Heterogeneity evaluation and meta-regression analysis
- There was between-study heterogeneity in sensitivity and specificity among the studies for F-18 FDG PET and PET/CT to detect recurrent disease after curative resection in patients with gastric cancer. Therefore, a meta-regression analysis was conducted on the selected studies to explore the potential reasons for this heterogeneity; however, no definite variable could be identified as the source of the observed heterogeneity (Table 2).
Results
- Although the development of surgical techniques, instruments, and chemo/radiotherapy has led to advances in cancer treatment using a multidisciplinary approach, the prognosis of advanced gastric cancer remains poor [4,33]. Traditionally, serum tumor markers (CEA and CA19-9), CT, and endoscopy have been used to detect recurrence, remnant gastric cancer, and metachronous cancer after surgery. Assessment of a tumor marker is relatively a cheap and convenient examination; however, it has low specificity for diagnostic confirmation and cannot locate the recurrence site. Endoscopy can identify lesions at the anastomotic site and remnant stomach; however, extraluminal recurrence cannot be detected [34]. Contrast-enhanced CT (CECT) is commonly used to diagnose primary and recurrent gastric cancer. It can accurately detect local recurrence or distant metastasis with high sensitivity. However, it has some limitations due to the distorted anatomical structure after surgery and its low specificity in cases of peritoneal spread [35]. However, F-18 FDG PET or PET/CT has the advantage of being less affected by anatomical distortion and provides functional details of organs and tissues, even at the cellular or molecular level.
- F-18 FDG PET or PET/CT, which relies on the glycolysis of cells with increased turnover, has been accepted as a valuable diagnostic tool capable of functional and anatomical evaluation of locoregional and distant recurrence, as well as staging [23]. Nevertheless, according to the Japanese gastric cancer treatment guideline and National Comprehensive Cancer Network guideline, F-18 FDG PET/CT is not a routine examination but is recommended in some clinically indicated cases, such as renal insufficiency or an allergy to a CT contrast agent [3].
- The current meta-analysis showed good sensitivity and specificity of F-18 FDG PET or PET/CT for detecting recurrent disease after curative resection of gastric cancer with 0.91 of the area under the curve in the receiver operating characteristic curve. For the detection of overall recurrence, F-18 FDG PET or PET/CT had a sensitivity of 82% (95% CI, 0.74–0.88) and specificity of 86% (95% CI, 0.78–0.91). This result confirmed that F-18 FDG PET or PET/CT is a useful diagnostic tool for evaluating recurrence or metastasis after curative gastrectomy.
- Several factors can affect the diagnostic accuracy of PET/CT. First, a sufficient tumor-to-background ratio cannot be obtained due to physiological uptake in the bowel [20]. Endoscopy may be necessary to evaluate small lesions within the lumen of the intestine. Second, small or flat lesions in peritoneal carcinomatosis can be challenging to detect because of low FDG uptake owing to the partial volume effect [9]. Although many studies reported similar results, we could not confirm this influence because we did not perform subgroup analysis for peritoneal carcinomatosis in this study [23,36,37]. Third, FDG uptake can be influenced by the histological type. SRC and MA have been shown to have low FDG avidity; however, the results of these studies have not been consistent [22,24,38]. In the current meta-analysis, the authors also confirmed the heterogeneity of FDG uptake in SRC and MA among the selected studies. However, SRC and MA were not significant factors influencing the diagnostic performance of PET/CT in the meta-regression analysis. Finally, benign pathological conditions or metabolically active mucosa of the gastrointestinal tract can increase physiological FDG uptake, which can decrease the specificity of F-18 FDG PET/CT. Involuntary smooth muscle contraction can also hide underlying peritoneal metastasis [20].
- Despite the high sensitivity and specificity of F-18 FDG PET or PET/CT in evaluating recurrence or metastasis, wide ranges have been observed in previous reports, with sensitivities from 43% to 100% and specificities from 53% to 100%. De Potter et al. [9] analyzed 33 patients with F-18 FDG PET alone and reported a sensitivity, specificity, and diagnostic accuracy of 70%, 69%, and 70%, respectively. Although F-18 FDG PET should not be the first tool for screening recurrence owing to its low diagnostic accuracy, FDG uptake showed a significant negative correlation with patient survival regardless of recurrence during the follow-up period. Lee et al. [23] compared the efficacy of F-18 FDG PET/CT with that of CECT in detecting the recurrence of gastric cancer after curative gastrectomy. The sensitivity and specificity were 42.9% and 59.7%, respectively, for F-18 FDG PET/CT; and 85.8% and 87.3%, respectively, for CECT. As the diagnostic accuracy for regional or distant lymph node metastasis in F-18 FDG PET/CT was 100%, it might show clinical usefulness. However, F-18 FDG PET/CT failed to show superiority in surveillance because the number of enrolled patients was small (n=21), and the diagnostic performance of F-18 FDG PET/CT was less accurate than that of CECT alone.
- In a recent study, Kim et al. [22] compared the efficacy of F-18 FDG PET/CT and CECT for detecting recurrence after curative gastrectomy. F-18 FDG PET/CT had a sensitivity, specificity, and accuracy of 82%, 95%, and 88%, respectively. All of these values were 95% for CECT, and there was no significant difference between the two methods (p=0.096). However, F-18 FDG PET/CT had a lower sensitivity for peritoneal metastasis than CECT (50% vs. 96%, p=0.001). These results are consistent with those of previous studies. Based on the pathological results, the specificity of F-18 FDG PET/CT was lower than that of CECT (80% vs. 98%, p=0.035) for tubular adenocarcinoma. In the SRC and MA groups, no significant differences between F-18 FDG PET/CT and CECT were found. In general, it has been shown in a previous study that SRC and MA have low FDG avidity [21]. However, the results were inconsistent with those of other studies. Through a meta-regression analysis, the results of the current study showed that SRC and MA did not affect the diagnostic performance of F-18 FDG PET/CT.
- Lee et al. [26] performed postoperative surveillance in 190 patients with gastric cancer using F-18 FDG PET/CT. Recurrence occurred in 19 patients; F-18 FDG PET/CT confirmed recurrence in 16 of them (8.4%). Seven of the 16 patients had extra-abdominal metastases, and the lesions could not be confirmed by conventional endoscopy or CECT. F-18 FDG PET/CT showed a sensitivity and specificity of 84.2% and 87.7%, respectively. The authors suggested that F-18 FDG PET/CT is an excellent diagnostic modality for asymptomatic recurrence, especially when CECT and endoscopy were not effective. However, the negative predictive value was 98.0%, the rate of false-positive findings was high (11.1%), and the positive predictive value was 43.2%. Therefore, the authors emphasized the need for a more careful examination.
- Kim et al. [20] analyzed the diagnostic accuracy of F-18 FDG PET/CT and CECT in 139 patients. Although the sensitivity of F-18 FDG PET/CT was relatively low (sensitivity, specificity, and diagnostic accuracy of 53.6%, 84.7%, and 78.4%, respectively), there were no significant differences between the values and those of CECT. The sensitivity, specificity, and diagnostic accuracy of F-18 FDG PET/CT in cases of distant metastasis were 100%, 98.5%, and 98.6%, respectively, (compared to 64.3%, 86.5%, and 82.0%, respectively, for CECT), suggesting that F-18 FDG PET/CT can be a valuable tool for surveillance after curative gastrectomy. The authors also reported that F-18 FDG PET/CT was less sensitive for detecting peritoneal metastasis than CECT (p=0.021). However, they emphasized that F-18 FDG PET/CT is a diagnostic tool as accurate as CECT and F-18 FDG PET/CT in addition to CECT could enhance the detection rate of recurrence and unexpected secondary malignancy.
- De Potter et al. [9], Jadvar et al. [19], and Yun et al. [32] evaluated the diagnostic accuracy of 18-FDG PET scans, and the accuracies were 69.7%, 83.3%, and 83.3%, respectively, which are acceptable values compared with the results of other PET/CT studies. Because the present study did not directly compare 18-FDG PET with PET/CT, a separate comparative analysis could not be conducted. Nakamoto et al. [27] compared 18-FDG PET and PET/CT and found that their diagnostic accuracies were 79% and 89%, respectively. Although the accuracy of PET/CT was slightly higher, both scans were excellent surveillance tools. Moreover, these results are consistent with those of previous studies. However, the authors reported that inline PET/CT is more advantageous than the manual fusion of PET and CT for anatomical localization.
- This meta-analysis examined the diagnostic potential of F-18 FDG PET/CT for detecting recurrence in patients who underwent curative gastrectomy. However, this study had several limitations. First, the diagnostic performance of F-18 FDG PET/CT to localize recurrence could not be analyzed owing to lack of data. Thirteen of the 17 studies reported recurrence locations; however, the proportions were heterogeneous. Although it is known that F-18 FDG PET/CT is less diagnostic than CECT, especially for peritoneal metastases, we were unable to conclude an association from this study. Second, not all patients who were TP were confirmed by histological examination, and the time intervals between the surgery and F-18 FDG PET or PET/CT were diverse. It would have been difficult to diagnose recurrence through histopathological confirmation in all patients and controls at the time intervals of the examinations. These factors may have biased the results. Third, the results revealed significant heterogeneity in sensitivity and specificity estimates across the included studies, likely due to methodological differences between the studies and patient populations. Furthermore, the considerable heterogeneity in the interpretation criteria for defining a positive PET scan was identified as a major limitation. While some studies employed visual evaluation, others used different quantitative indices to interpret the F-18 FDG PET or PET/CT images. Moreover, the criteria used to diagnose microvascular invasion were not standardized across the included studies. To minimize bias, independent reviewers (blinded to the journal, author, institution, and publication date) selected articles based on predefined inclusion criteria and used a standardized form based on the QUADAS-2 tool to evaluate study design characteristics and examination results. Despite our efforts to minimize bias, publication bias remains a concern as studies with significant findings are more likely to be published. Overall, the present meta-analysis demonstrated good sensitivity and specificity of F-18 FDG PET or PET/CT for detecting disease recurrence after curative resection of gastric cancer. However, owing to the limited literature in this area, further large multicenter studies are required to confirm the diagnostic accuracy of F-18 FDG PET and PET/CT in detecting recurrence after curative resection of gastric cancer.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
This work was supported by Pusan National University Research Grant, 2022 (No. 202207880001).
-
Author contributions
Conceptualization, Formal analysis: all authors; Data curation: JKP, DHK; Funding acquisition: DHK; Methodology: JKP, TYJ; Supervision: TYJ, DHK; Writing-original draft: CIC; Writing-review & editing: TYJ, DHK.
Notes
| Study | Year | Country | Study design | Gold standard |
Patient number |
Sex, male/female | Age (yr), mean (range) | Dose of F-18 FDG (MBq) | Interpretation criteria of PET | Cutoff | SRC, MA (%) | Recurrence (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PET | PET/CT | ||||||||||||
| Bilici et al. [8] | 2011 | Turkey | Retrospective | HP and C-F/U | 34 | 27/7 | 58.5 (32-79) | 370 | QA | 3.0 | 23.5 | 70.6 | |
| De Potter et al. [9] | 2002 | Belgium | Retrospective | HP and C-F/U | 33 | 23/10 | 58.5 | 6.5 MBq/kg | VA | 20.0 | 60.6 | ||
| Graziosi et al. [18] | 2011 | Italy | Retrospective | HP and C-F/U | 50 | NR | 68.4 | 4.5 MBq/kg | VA | NR | 58.0 | ||
| Jadvar et al. [19] | 2003 | USA | Retrospective | HP and C-F/U | 18 | 12/6 | 60.4 (37-79) | 370 | VA | 11.2 | 61.1 | ||
| Kim et al. [20] | 2011 | Korea | Retrospective | HP and C-F/U | 139 | 88/51 | 61.5 (60-64) | 370 | VA | 13.2 | 20.1 | ||
| Kim et al. [21] | 2016 | Korea | Retrospective | HP and C-F/U | 368 | 250/118 | 57.8 | 5 MBq/kg | QA | 4.0 | 13.9 | 19.6 | |
| Kim et al. [22] | 2017 | Korea | Retrospective | HP and C-F/U | 120 | 53/67 | 62.5 | 7.4 MBq/kg | QA | 3.0 | 8.3 | 50.0 | |
| Lee et al. [23] | 2011 | Korea | Retrospective | HP and C-F/U | 89 | 62/27 | 56.4 (27-82) | 360 | VA | 26.9 | 16.8 | ||
| Lee et al. [24] | 2012 | Korea | Retrospective | HP and C-F/U | 271 | 171/100 | 60 | 5.2 MBq/kg | VA | 11.4 | 17.3 | ||
| Lee et al. [25] | 2014 | Korea | Retrospective | HP and C-F/U | 46 | 29/17 | 60.6 | 370 | VA | 10.8 | 8.7 | ||
| Lee et al. [26] | 2016 | Korea | Retrospective | HP and C-F/U | 190 | 126/64 | 61 (29-80) | 5.2 MBq/kg | VA | 5.8 | 8.4 | ||
| Nakamoto et al. [27] | 2009 | Japan | Retrospective | HP and C-F/U | 47 | 45 | 61/31 | 67 (31-87) | 370 | VA | 16.3 | 47.8 | |
| Park et al. [28] | 2009 | Korea | Retrospective | HP and C-F/U | 105 | 75/30 | 58 (34-83) | 370 | VA | 24.7 | 71.4 | ||
| Sharma et al. [29] | 2012 | India | Retrospective | HP and C-F/U | 72 | 52/20 | 52.8 (28-86) | 370 | VA | NR | 68.1 | ||
| Sim et al. [30] | 2009 | Korea | Retrospective | HP and C-F/U | 52 | 43/9 | 62 (33-80) | 555 | VA | 7.7 | 73.1 | ||
| Sun et al. [31] | 2008 | China | Retrospective | HP and C-F/U | 23 | 15/8 | 55.4 | 370 | VA | 35.7 | NR | ||
| Yun et al. [32] | 2005 | Korea | Retrospective | HP and C-F/U | 30 | 22/8 | 58.3 (27-80) | NR | VA | 13.3 | 56.6 | ||
| Variable | Coefficienta) | SE | DOR | 95% CI of DOR | p-valueb) |
|---|---|---|---|---|---|
| Ethnicity, Caucasian vs. Asianc) | 0.610 | 1.1756 | 1.84 | 0.14–24.47 | 0.6141 |
| Recurrence (+), >53.3% vs. ≤53.3% | –0.139 | 0.9776 | 0.87 | 0.10–7.48 | 0.8893 |
| SRC and MA, >13.3% vs. ≤13.3% | –0.422 | 0.7624 | 0.66 | 0.12–3.51 | 0.5911 |
| Interpretation criteria, quantitative vs. visuald) | 2.444 | 1.1544 | 11.52 | 0.91–146.21 | 0.0578 |
SE, standard error; DOR, diagnostic odds ratio; CI, confidence interval; SRC, signet ring cell carcinoma; MA, mucinous adenocarcinoma.
a) Regression coefficient.
b) p-value of random effects meta-regression using maximum likelihood estimation between study variances and weighted least squares of study size for regression model estimation.
c) 1, Caucasian vs. 0, Asian;
d) 1, quantitative vs. 0, visual.
- 1. International Agency for Research on Cancer. Cancer fact sheet stomach: Globocan 2020 [Internet]. Geneva: World Health Organization; 2022 [cited 2023 Apr 30]. https://gco.iarc.fr/today/data/factsheets/cancers/7-Stomach-fact-sheet.pdf.
- 2. National Cancer Center; Korea Central Cancer Registry. Korean Cancer Report 2020. Goyang: National Cancer Center; 2022.
- 3. Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023;26:1–25.ArticlePubMedPDF
- 4. Park SH, Kang MJ, Yun EH, Jung KW. Epidemiology of Gastric Cancer in Korea: Trends in Incidence and Survival Based on Korea Central Cancer Registry Data (1999-2019). J Gastric Cancer 2022;22:160–8.ArticlePubMedPMCPDF
- 5. Blodgett TM, Meltzer CC, Townsend DW. PET/CT: form and function. Radiology 2007;242:360–85.ArticlePubMed
- 6. Czernin J, Allen-Auerbach M, Schelbert HR. Improvements in cancer staging with PET/CT: literature-based evidence as of September 2006. J Nucl Med 2007;48(1 Suppl):78S–88S.PubMed
- 7. Bar-Shalom R, Yefremov N, Guralnik L, Gaitini D, Frenkel A, Kuten A, et al. Clinical performance of PET/CT in evaluation of cancer: additional value for diagnostic imaging and patient management. J Nucl Med 2003;44:1200–9.PubMed
- 8. Bilici A, Ustaalioglu BB, Seker M, Kefeli U, Canpolat N, Tekinsoy B, et al. The role of 18F-FDG PET/CT in the assessment of suspected recurrent gastric cancer after initial surgical resection: can the results of FDG PET/CT influence patients' treatment decision making? Eur J Nucl Med Mol Imaging 2011;38:64–73.ArticlePubMedPDF
- 9. De Potter T, Flamen P, Van Cutsem E, Penninckx F, Filez L, Bormans G, et al. Whole-body PET with FDG for the diagnosis of recurrent gastric cancer. Eur J Nucl Med Mol Imaging 2002;29:525–9.ArticlePubMedPDF
- 10. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36.ArticlePubMed
- 11. Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. J Clin Epidemiol 2003;56:1129–35.ArticlePubMed
- 12. Thompson SG. Why sources of heterogeneity in meta-analysis should be investigated. BMJ 1994;309:1351–5.ArticlePubMedPMC
- 13. Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 2005;58:882–93.ArticlePubMed
- 14. Hamza TH, van Houwelingen HC, Stijnen T. The binomial distribution of meta-analysis was preferred to model within-study variability. J Clin Epidemiol 2008;61:41–51.ArticlePubMed
- 15. Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982–90.ArticlePubMed
- 16. Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med 2001;20:2865–84.ArticlePubMed
- 17. Lijmer JG, Mol BW, Heisterkamp S, Bonsel GJ, Prins MH, van der Meulen JH, et al. Empirical evidence of design-related bias in studies of diagnostic tests. JAMA 1999;282:1061–6.ArticlePubMed
- 18. Graziosi L, Bugiantella W, Cavazzoni E, Cantarella F, Porcari M, Baffa N, et al. Role of FDG-PET/CT in follow-up of patients treated with resective gastric surgery for tumour. Ann Ital Chir 2011;82:125–9.PubMed
- 19. Jadvar H, Tatlidil R, Garcia AA, Conti PS. Evaluation of recurrent gastric malignancy with [F-18]-FDG positron emission tomography. Clin Radiol 2003;58:215–21.ArticlePubMed
- 20. Kim DW, Park SA, Kim CG. Detecting the recurrence of gastric cancer after curative resection: comparison of FDG PET/CT and contrast-enhanced abdominal CT. J Korean Med Sci 2011;26:875–80.ArticlePubMedPMCPDF
- 21. Kim SJ, Cho YS, Moon SH, Bae JM, Kim S, Choe YS, et al. Primary tumor ¹⁸F-FDG avidity affects the performance of ¹⁸F-FDG PET/CT for detecting gastric cancer recurrence. J Nucl Med 2016;57:544–50.ArticlePubMed
- 22. Kim JH, Heo SH, Kim JW, Shin SS, Min JJ, Kwon SY, et al. Evaluation of recurrence in gastric carcinoma: comparison of contrast-enhanced computed tomography and positron emission tomography/computed tomography. World J Gastroenterol 2017;23:6448–56.ArticlePubMedPMC
- 23. Lee JE, Hong SP, Ahn DH, Jeon TJ, Kang MK, Kwon CI, et al. The role of 18F-FDG PET/CT in the evaluation of gastric cancer recurrence after curative gastrectomy. Yonsei Med J 2011;52:81–8.ArticlePubMed
- 24. Lee JW, Lee SM, Lee MS, Shin HC. Role of ¹⁸F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Imaging 2012;39:1425–34.ArticlePubMedPDF
- 25. Lee DY, Lee CH, Seo MJ, Lee SH, Ryu JS, Lee JJ. Performance of (18)F-FDG PET/CT as a postoperative surveillance imaging modality for asymptomatic advanced gastric cancer patients. Ann Nucl Med 2014;28:789–95.ArticlePubMedPDF
- 26. Lee JW, Lee SM, Son MW, Lee MS. Diagnostic performance of FDG PET/CT for surveillance in asymptomatic gastric cancer patients after curative surgical resection. Eur J Nucl Med Mol Imaging 2016;43:881–8.ArticlePubMedPDF
- 27. Nakamoto Y, Togashi K, Kaneta T, Fukuda H, Nakajima K, Kitajima K, et al. Clinical value of whole-body FDG-PET for recurrent gastric cancer: a multicenter study. Jpn J Clin Oncol 2009;39:297–302.ArticlePubMed
- 28. Park MJ, Lee WJ, Lim HK, Park KW, Choi JY, Kim BT. Detecting recurrence of gastric cancer: the value of FDG PET/CT. Abdom Imaging 2009;34:441–7.ArticlePubMedPDF
- 29. Sharma P, Singh H, Suman SK, Sharma A, Reddy RM, Thulkar S, et al. 18F-FDG PET-CT for detecting recurrent gastric adenocarcinoma: results from a Non-Oriental Asian population. Nucl Med Commun 2012;33:960–6.ArticlePubMed
- 30. Sim SH, Kim YJ, Oh DY, Lee SH, Kim DW, Kang WJ, et al. The role of PET/CT in detection of gastric cancer recurrence. BMC Cancer 2009;9:73.ArticlePubMedPMCPDF
- 31. Sun L, Su XH, Guan YS, Pan WM, Luo ZM, Wei JH, et al. Clinical role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in post-operative follow up of gastric cancer: initial results. World J Gastroenterol 2008;14:4627–32.ArticlePubMedPMC
- 32. Yun M, Choi HS, Yoo E, Bong JK, Ryu YH, Lee JD. The role of gastric distention in differentiating recurrent tumor from physiologic uptake in the remnant stomach on 18F-FDG PET. J Nucl Med 2005;46:953–7.PubMed
- 33. Lee JH, Son SY, Lee CM, Ahn SH, Park DJ, Kim HH. Factors predicting peritoneal recurrence in advanced gastric cancer: implication for adjuvant intraperitoneal chemotherapy. Gastric Cancer 2014;17:529–36.ArticlePubMedPDF
- 34. Whiting J, Sano T, Saka M, Fukagawa T, Katai H, Sasako M. Follow-up of gastric cancer: a review. Gastric Cancer 2006;9:74–81.ArticlePubMedPDF
- 35. Kim KW, Choi BI, Han JK, Kim TK, Kim AY, Lee HJ, et al. Postoperative anatomic and pathologic findings at CT following gastrectomy. Radiographics 2002;22:323–36.ArticlePubMed
- 36. Lim JS, Kim MJ, Yun MJ, Oh YT, Kim JH, Hwang HS, et al. Comparison of CT and 18F-FDG pet for detecting peritoneal metastasis on the preoperative evaluation for gastric carcinoma. Korean J Radiol 2006;7:249–56.ArticlePubMedPMC
- 37. Wu LM, Hu JN, Hua J, Gu HY, Zhu J, Xu JR. 18 F-fluorodeoxyglucose positron emission tomography to evaluate recurrent gastric cancer: a systematic review and meta-analysis. J Gastroenterol Hepatol 2012;27:472–80.ArticlePubMed
- 38. Alakus H, Batur M, Schmidt M, Drebber U, Baldus SE, Vallböhmer D, et al. Variable 18F-fluorodeoxyglucose uptake in gastric cancer is associated with different levels of GLUT-1 expression. Nucl Med Commun 2010;31:532–8.ArticlePubMed
References
Figure & Data
References
Citations


 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine
 PubReader
PubReader ePub Link
ePub Link Cite
Cite