PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(Suppl); 2023 > Article
-
Original article
Comparison of serum anti-Müllerian hormone between unilateral and bilateral ovarian endometriomas during follicular, luteal, and random menstrual phases: a retrospective study -
Juhun Lee1
 , Jong Mi Kim2
, Jong Mi Kim2 , Gun Oh Chong2
, Gun Oh Chong2 , Dae Gy Hong2
, Dae Gy Hong2 , Yoon Hee Lee2
, Yoon Hee Lee2
-
Journal of Yeungnam Medical Science 2023;40(Suppl):S65-S72.
DOI: https://doi.org/10.12701/jyms.2023.00661
Published online: September 22, 2023
1Department of Obstetrics and Gynecology, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu, Korea
2Department of Obstetrics and Gynecology, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, Daegu, Korea
- Corresponding author: Yoon Hee Lee, MD Department of Obstetrics and Gynecology, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, 807 Hoguk-ro, Buk-gu, Daegu 41404, Korea Tel: +82-53-200-2167 • Fax: +82-53-200-2681 • E-mail: yhlee1017@knu.ac.kr
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Over the last two decades, serum levels of anti-Müllerian hormone (AMH) have been shown to be reliable markers of ovarian reserve. This study aimed to compare baseline serum AMH levels and well-controlled clinical factors between patients with unilateral and bilateral ovarian endometriomas during the menstrual phase.
-
Methods
- We conducted a retrospective study. We enrolled 136 patients aged 18 to 36 years who were diagnosed with unilateral or bilateral ovarian endometriomas. Serum AMH levels of all patients and their latest two to three menstrual cycles were measured before surgery for ovarian endometriomas. The latest menstrual cycle length ranged from 26 to 30 days. Patients with irregular menstruation, a recent medication history of hormonal drugs other than oral contraceptive pills, a previous history of ovarian surgery, or any medical history influencing ovarian function were excluded.
-
Results
- Of the 136 patients, 76 (55.9%) had unilateral ovarian endometriomas and 60 (44.1%) had bilateral ovarian endometriomas. Serum AMH levels were not significantly different between the two groups in the follicular phase, luteal phase, or at any random time point.
-
Conclusion
- Serum AMH levels were not significantly different between unilateral and bilateral ovarian endometriomas in the follicular and luteal phases, or at any random time during the menstrual cycle when various confounding factors were excluded.
- Over the last two decades, serum levels of anti-Müllerian hormone (AMH) have been known as reliable markers of ovarian reserve [1,2]. Several studies have demonstrated relatively lower AMH variability across the menstrual cycle compared with other serum markers, such as follicle-stimulating hormone and estradiol [1,3]. Although serum AMH levels are relatively stable, they vary throughout the follicular and luteal phases [4-6]. However, owing to the limited number of participants in previous studies, the variability in AMH levels across menstrual phases has not been firmly established.
- In a recent meta-analysis, patients with ovarian endometriomas showed significantly lower AMH levels than those with other benign ovarian cysts or normal ovaries [7]. Several well-supported molecular hallmarks have been identified in the pathophysiology of endometriosis, including genetic predisposition, estrogen dependence, progesterone resistance, and inflammation [8]. However, the mechanism underlying the decrease in AMH levels in endometriosis has not yet been fully elucidated. Intuitively, patients with bilateral ovarian endometriomas are expected to have significantly lower serum AMH levels than patients with unilateral endometriomas. Although many previous reports have shown lower serum AMH levels after ovarian surgery in patients with bilateral ovarian endometriomas than in those with unilateral endometrioma, many of these reports did not compare baseline serum AMH levels between the two groups. In a previous study with a large number of participants, significantly lower AMH levels were found in bilateral endometriomas [9]. However, in recent studies, controversial results have been reported with a small number of participants [10,11].
- In previous studies comparing the baseline AMH levels between unilateral and bilateral endometriomas, the authors did not show whether patients with an irregular menstrual cycle, previous history of ovarian surgery, or those who were 40 years and older were excluded. Furthermore, the variability in AMH levels across the menstrual phases, including the follicular and luteal phases, was not considered. In the present study, we aimed to compare baseline serum AMH levels between patients with unilateral and bilateral ovarian endometriomas, considering the menstrual phase and controlling for relevant clinical factors.
Introduction
- Ethical statements: This study was approved by the Institutional Review Board (IRB) of Kyungpook National University Chilgok Hospital (IRB No: KNUCH 2022-09-006), and the requirement for informed consent was waived.
- 1. Patients and treatment
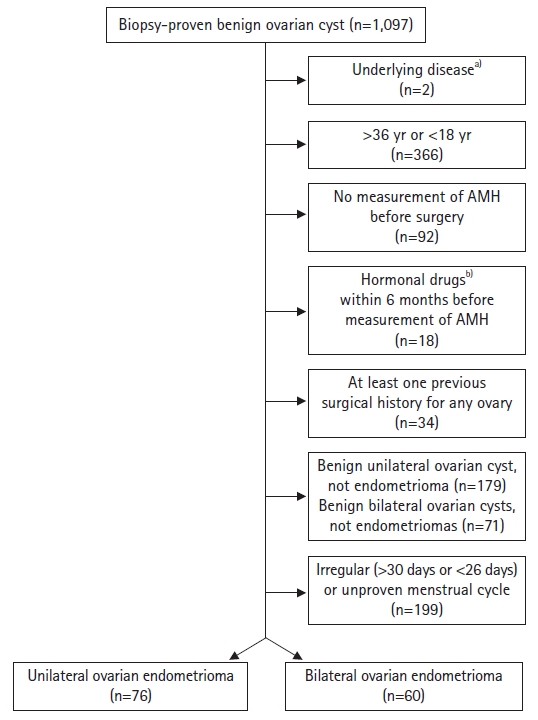
- The medical records of 1,097 patients diagnosed with benign ovarian cysts determined by pathological examination of intraoperative specimens from January 2012 to July 2022 at Kyungpook National University Chilgok Hospital were used. Two patients were excluded because of underlying disease or medical history, that is, premature ovarian failure and being a liver transplantation donor, respectively. Patients taking thyroid hormone medications with normal thyroid function test results were included in the study. A total of 366 patients were excluded because of their age, that is, those older than 36 years or younger than 18 years at the time of AMH measurement. Furthermore, 92 patients were excluded because their serum AMH levels were not measured. We also excluded 18 patients due to the use of gonadotropin-releasing hormone agonists or dienogest within 6 months prior to AMH measurement. However, only three patients who used oral contraceptives (OCs) were included. In addition, 34 patients with a previous history of at least one ovarian surgery were excluded, and only those with unilateral or bilateral ovarian endometriomas were included. Therefore, 250 patients were excluded from the study. Of the remaining patients, 199 with irregular or unknown menstrual cycles were excluded, and only those with a menstrual cycle length of 26 to 30 days were included. Ultimately, 136 patients were enrolled (Fig. 1).
- 2. Definition of normal menstrual cycle
- Each patient recorded her two latest menstrual periods before serum AMH level measurement and ovarian surgery. During hospital admission, each patient recorded her latest menstrual period again so that two or three latest menstrual periods could be reviewed. Only patients with regular menstrual cycles of 26 to 30 days were included in the study.
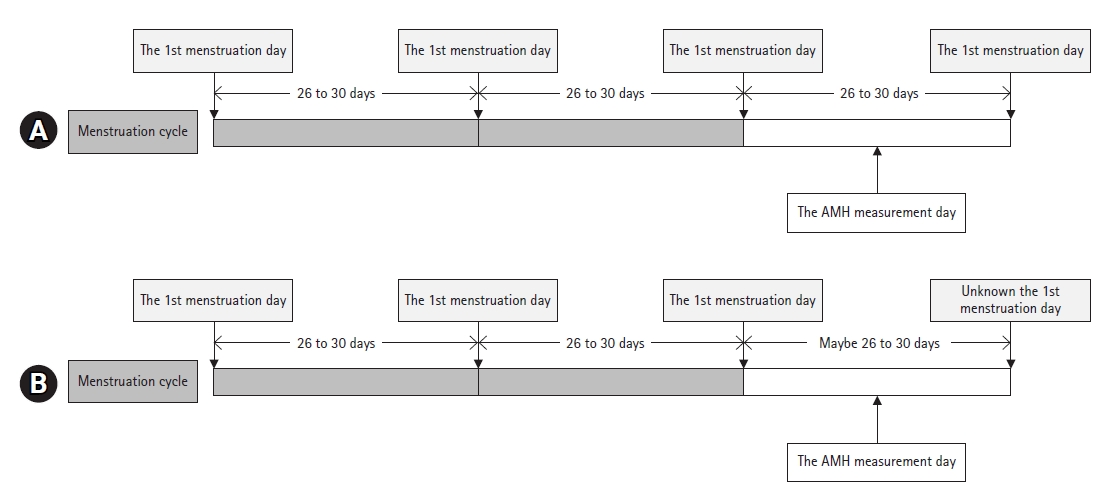
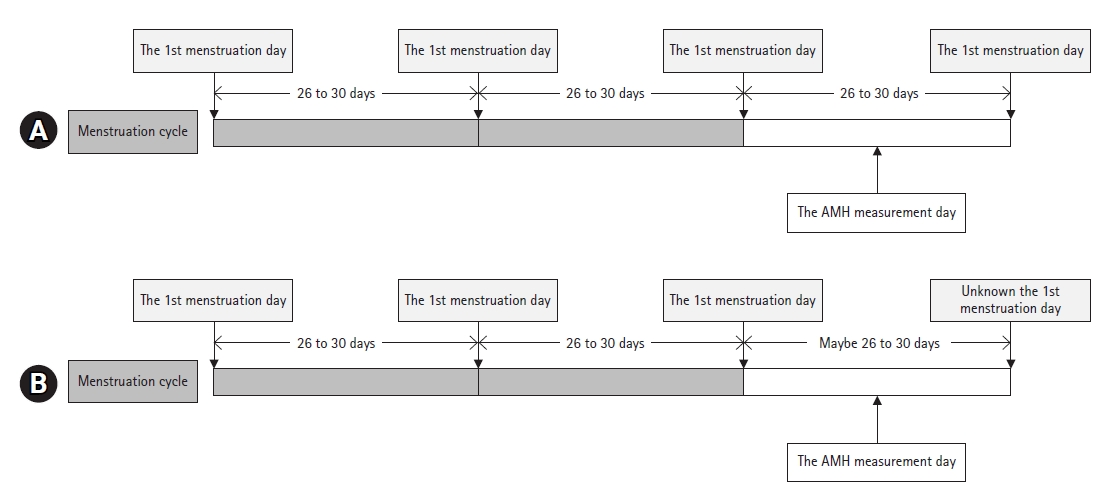
- The lengths of the follicular or luteal phases were determined using an equation from a previous study [12]. Patients were categorized into two types: (1) patients whose first menstruation day before and after AMH level measurement (Fig. 2A) could be ascertained and (2) patients whose menstrual cycle length could not be ascertained after AMH level measurement, but the two regular (26–30 days) and latest menstrual cycles before AMH measurement (Fig. 2B) could be identified. The length of the last menstrual cycle (white bar in Fig. 2) was used to calculate the follicular phase length. The following equations were used to calculate the duration of each menstrual phase in each group:
- 1) Follicular phase length=0.770×menstruation cycle length−7.685
- 2) Luteal phase length=menstrual cycle length−follicular phase length
- 3. Measurement of serum anti-Müllerian hormone
- The chemiluminescence immunoassay Unicel DxI 800 (Beckman Coulter; Fullerton, CA, USA) was used to measure serum AMH levels according to the manufacturer’s instructions. This method had a detection threshold of 0.035 pmol/L (0.0049 ng/mL) and a variation coefficient of ≤10.0% for an AMH of ≥0.16 ng/mL.
- 4. Other clinical factors
- The patients were examined using abdominal computed tomography (CT) or magnetic resonance imaging (MRI) of the pelvis before surgery, except for three patients. We measured the largest ovarian endometrioma diameter on the axial or coronal images. For the three patients who did not undergo pelvic CT or MRI, the lesion size was measured using pelvic ultrasound. Blood inflammatory markers, such as cancer antigen 125 (CA125, U/mL), C-reactive protein (CRP, mg/dL), and erythrocyte sedimentation rate (ESR, mm/hr), were measured within 30 days from the day of measuring AMH in all patients. Some patients were measured before AMH measurement, others on the same day, and the rest were after AMH measurement. The disease stage was determined according to the revised classification of endometriosis by the American Society for Reproductive Medicine [13]. Dysmenorrhea was evaluated using a numerical rating system (NRS). Body mass index (BMI) was defined as weight divided by height squared (kg/m2).
- 5. Statistical analysis
- To compare continuous variables between the groups, the Student t-test was used if the number in each group was more than 30 or showed a standard normal distribution, and the Mann-Whitney U-test was used if the above criteria were not satisfied. To compare categorical variables between the groups, the chi-square test or Fisher exact test was used. Multivariate regression analysis was used to evaluate the correlation between clinical factors in subgroup analysis. All statistical analyses were performed using IBM SPSS ver. 26 (IBM Corp., Armonk, NY, USA).
Methods
- 1. Baseline characteristics
- Of the 136 patients, 76 (55.9%) had unilateral ovarian endometriomas and 60 (44.1%) had bilateral ovarian endometriomas. The mean age was 28.87±4.09 and 28.65±4.82 years, respectively. The mean menstrual cycle length was 28.12±1.44 and 28.30±1.41 days, respectively. The mean follicular phase length was 13.86±1.13 and 14.08±1.12 days, respectively. The mean luteal phase length was 14.26±0.44 and 14.22±0.45 days, respectively. Three patients (3.9%) with unilateral endometriomas used OCs within 6 months before AMH level measurement, whereas none of the patients with bilateral endometriomas used OCs. The mean BMIs of the unilateral and bilateral groups were 20.58±2.91 and 21.56±4.06 kg/m2, respectively. The mean NRS scores for dysmenorrhea in the unilateral and bilateral groups were 4.46±2.86 and 5.37±2.50, respectively. None of these clinical factors differed significantly. When comparing the unilateral and bilateral groups, the gravidity, parity, and number of abortions were 0.03±0.16 vs. 0.25±0.65 (p=0.012), 0.03±0.16 vs. 0.17±0.53 (p=0.050), and 0.00±0.00 vs. 0.08±0.28 (p=0.024), respectively. The proportion of patients with stage IV disease was significantly lower in the unilateral group (40 [52.6%] vs. 52 [86.7%], p<0.001). The total size of the ovarian endometrioma was significantly smaller in the unilateral group (5.99±2.36 cm vs. 9.42±3.28 cm, p<0.001). Although CRP and CA125 levels were not significantly different between the unilateral and bilateral groups CRP: 0.22±0.64 mg/dL vs. 0.32±0.83 mg/dL, respectively, p=0.393; CA125: 81.70±138.22 U/mL vs. 87.57±70.77 U/mL, respectively, p=0.765), the ESR was significantly lower in patients with unilateral endometriomas (15.33±14.99 mm/hr vs. 21.68±17.29 mm/hr, p=0.024) (Table 1).
- 2. Comparison of serum levels of anti-Müllerian hormone
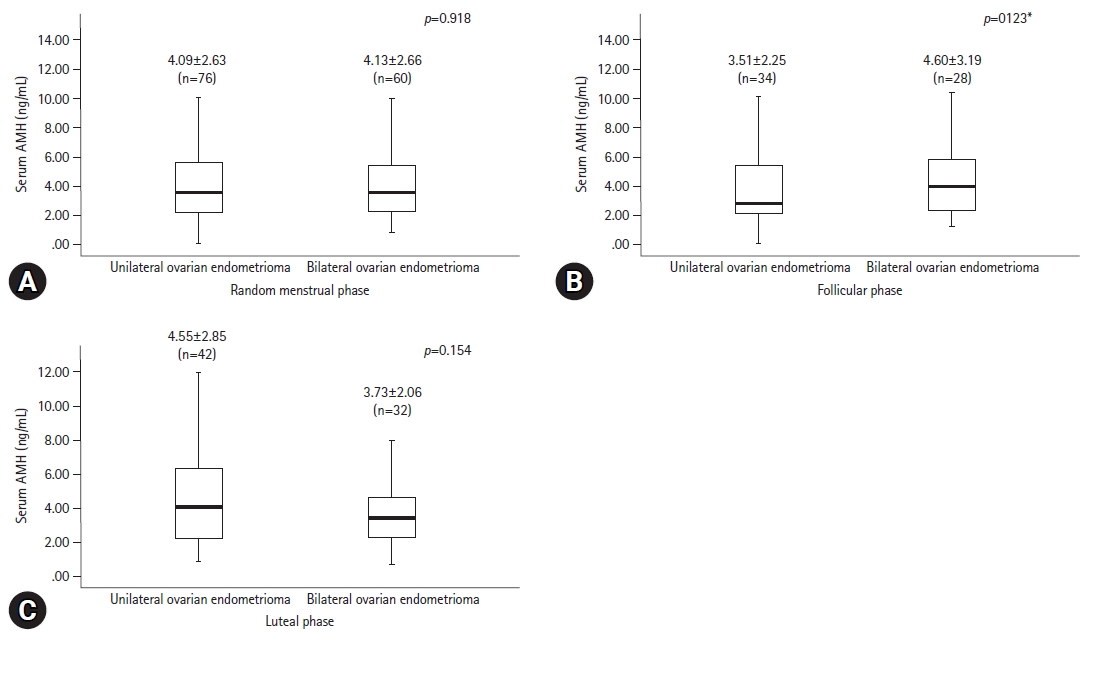
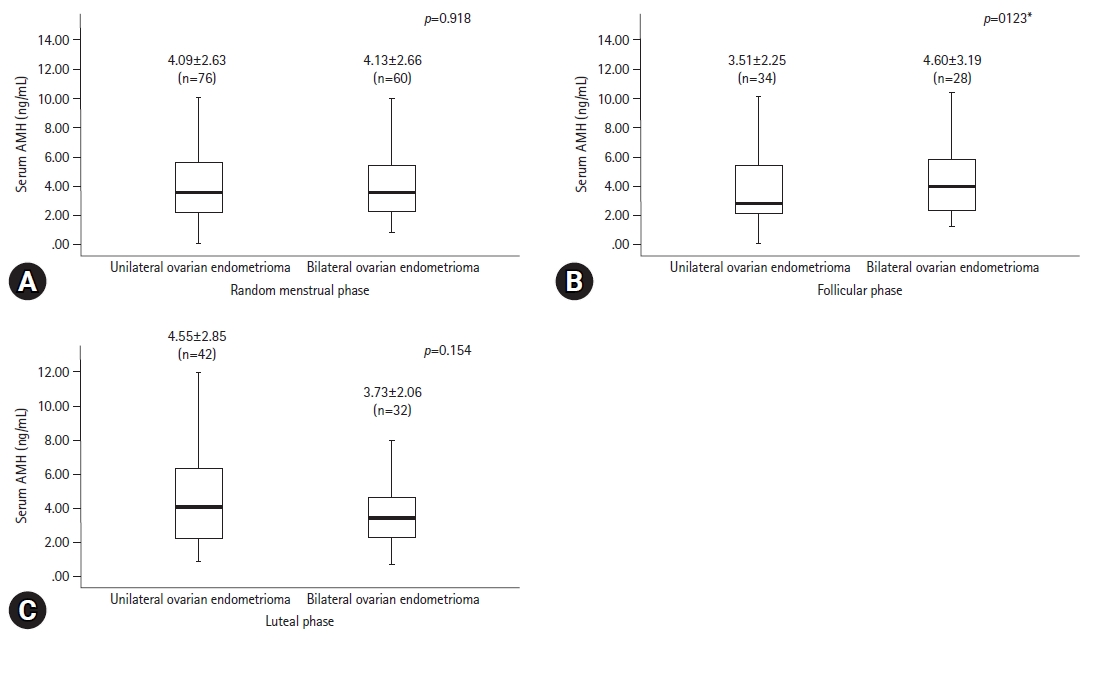
- The serum levels of AMH, which were randomly measured during the menstrual phase, were not significantly different between the unilateral and bilateral endometrioma groups (4.09±2.63 ng/mL vs. 4.13±2.66 ng/mL, respectively, p=0.918). Of the 62 patients in whom AMH levels were measured in the follicular phase group, 34 (54.8%) had unilateral and 28 (45.2%) had bilateral endometriomas. AMH serum levels in the follicular phase group were 3.51±2.25 ng/mL and 4.60±3.19 ng/mL, respectively, which were not significantly different (p=0.123). Of the 74 patients whose AMH levels were measured in the luteal phase, 42 (56.8%) had unilateral and 32 (43.2%) had bilateral endometriomas with AMH levels of 4.55±2.85 ng/mL and 3.73±2.06, respectively, which were not significantly different (p=0.154) (Fig. 3). No significant differences in AMH levels were observed after adjusting for baseline characteristics that were significantly different, including number of abortions, disease stage, and endometrioma size.
Results
- Serum AMH levels in unilateral and bilateral ovarian endometriomas were not significantly different when measured randomly during the menstrual cycle.
- AMH levels were not higher in the unilateral ovarian endometrioma group, which had one more intact ovary, than in the bilateral endometriomas group. This finding suggests that ovarian endometrioma may result in decreased serum AMH levels via an indirect pathway rather than a direct pathway, such as the destruction of AMH-producing cells or tissues. The mechanism by which endometriosis or ovarian endometrioma decreases serum AMH levels has not yet been elucidated. Inflammation is a potential factor in endometriosis pathophysiology [8]. Inflammation may be an indirect cause of decreased AMH levels in patients with endometriosis compared with the levels in patients without endometriosis. An inflammatory condition due to endometriosis might have suppressed the increase in AMH; this has also been reported previously in a study elucidating the correlation between AMH levels and disease activity in Crohn disease [14]. In the present study, the bilateral ovarian endometrioma group included a significantly greater number of patients with stage IV disease and showed a significantly increased ESR among the three blood markers of inflammation. Although we found a recent study that reported an increase in ESR correlated with endometriosis severity, the study included only a small number of patients, and in contrast to ESR, CA125 levels were not significantly different in our study [15]. ESR may be a more sensitive blood marker of endometriosis severity; however, more reliable evidence is required.
- Our study had several strengths. We excluded biases that could influence AMH, such as age of <18 and >36 years, hormonal medication prior to measurement, irregular menstruation, and previous history of ovarian surgery. Additionally, the analysis was conducted on a homogenous cohort. AMH levels were not only evaluated in each of the two menstrual phases (follicular and luteal) but also randomly during the menstrual cycle.
- However, this study also had some limitations. First, aside from the small number of patients, this was a retrospective study with data from a single center. Second, ovulation or luteinizing hormone surge was not established. The duration of the follicular or luteal phase was determined using an equation from a previous study. Third, the variation in serum AMH levels across the menstrual cycle in individual patients was not considered in this study. Fourth, we were unable to review certain inflammatory factors that reflect local inflammatory conditions in the pelvis. There are many known molecular markers of inflammation in endometriosis, such as interleukins or prostaglandins [16]. Although Kitajima et al. failed to find homogenous changes in a recent study, analyzing molecular markers in peritoneal fluid or wash can be meaningful [17]. Finally, we did not evaluate the effect of OCs on AMH levels. Although OCs can affect AMH levels [18], we could not exclude this because anyone can use OCs without a prescription in Korea.
- We conducted a subgroup analysis to evaluate the correlation between clinical factors and AMH according to menstrual phase. All clinical factors were analyzed using multivariate linear regression for follicular and luteal phases in the total, unilateral, and bilateral endometrioma groups. Age was a significant factor in the luteal phase subgroup of the total, unilateral, and bilateral endometrioma groups (p=0.007, p=0.013, and p=0.008, respectively); the regression coefficient (β) was −0.297, −0.366, and −0.441, respectively. The length of the follicular phase was a significant factor in the follicular phase subgroup of the total group (p=0.003, β=0.283). The length of the luteal phase was a significant factor in the luteal phase subgroup of the total group (p=0.001, β=0.358). BMI was a significant factor in the luteal phase subgroup of the bilateral endometrioma group (p=0.044, β=0.314). The NRS of dysmenorrhea was a significant factor in the luteal phase subgroup of the unilateral endometrioma group (p=0.017, β=0.352). None of the blood inflammatory markers (CRP, ESR, or CA125) was found to be a significant factor (Table 2). In these subgroup analyses, age appeared to have a partially negative effect on serum AMH levels in the luteal phase, and the length of the menstrual phase seemed to be correlated. To date, an inverse correlation between AMH levels and age during the menstrual phase has not been reported. Numerous studies have demonstrated that AMH levels decrease with age [19]. No previous reports have elucidated the relationship between AMH levels and menstrual phase length. Therefore, we could not establish a hypothesis for these findings. For BMI, a previous study indicated that females with a BMI over 25 kg/m2 showed elevated AMH levels during the luteal phase, which is in contrast to the follicular phase, where decreased levels were observed [5]. Some authors have found an inverse correlation between BMI and AMH levels [18,19]. However, the exact mechanism underlying this relationship was not described in those studies. Our study included a small number of females who were obese, which was insufficient to evaluate the relationship between obesity and AMH levels. The dysmenorrhea score was not considered a significant factor for AMH levels because it was dependent on the patient's subjective quantification. The total endometrioma size did not show a highly significant correlation with AMH levels, unlike in previous studies [9,11]. This finding may support our interpretation that ovarian endometriomas do not directly inhibit granulosa cells where AMH is produced.
- We conducted another subgroup analysis to evaluate AMH variations across the follicular or luteal phases using linear regression. Previous studies have reported a tendency of increased AMH levels in the late follicular phase [4,5]; however, we did not find a significant tendency across each phase. We also compared the mean AMH level between the follicular and luteal phases in the total, unilateral endometrioma, and bilateral endometrioma groups (4.00±2.75 ng/mL vs. 4.19±2.56 ng/mL, p=0.676; 3.51±2.25 ng/mL vs. 4.55±2.85 ng/mL, p=0.088; and 4.60±3.19 vs. 3.73±2.06 ng/mL, p=0.209, respectively), and no significant differences were observed. Therefore, these findings provide evidence that AMH is a reliable marker, regardless of the menstrual phase. A study with a large number of participants is warranted to further evaluate variations across the menstrual cycle or phase because these subgroups were too small for proper analysis in our study.
- In conclusion, serum AMH levels were not significantly different between unilateral and bilateral ovarian endometriomas in the follicular phase, luteal phase, or at any random time in the menstrual cycle when various confounding factors were excluded.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
-
Author contributions
Conceptualization: YHL, GOC, DGH, JL; Data curation: YHL, JMK, JL; Formal analysis: YHL, GOC, DGH; Validation: GOC; Methodology, Software: YHL; Project administration, Resources: JL; Investigation: YHL, JL; Supervision: GOC, DGH; Visualization: JMK, JL; Writing-original draft: all authors; Writing-review & editing: all authors.
Notes
| Characteristic | Ovarian endometrioma | ||
|---|---|---|---|
| Unilateral | Bilateral | p-value | |
| No. of patients | 76 (55.9) | 60 (44.1) | |
| Age (yr) | 28.87±4.09 | 28.65±4.82 | 0.780 |
| Length of menstrual cycle (day) | 28.12±1.44 | 28.30±1.41 | 0.462 |
| Length of follicular phasea) (day) | 13.86±1.13 | 14.08±1.12 | 0.243 |
| Length of luteal phaseb) (day) | 14.26±0.44 | 14.22±0.45 | 0.549 |
| Gravidity (time) | 0.03±0.16 | 0.25±0.65 | 0.012 |
| Parity (time) | 0.03±0.16 | 0.17±0.53 | 0.050 |
| Abortion (time) | 0.00±0.00 | 0.08±0.28 | 0.024 |
| Body mass index (kg/m2) | 20.58±2.91 | 21.56±4.06 | 0.106 |
| Stage by rASRM | <0.001 | ||
| I | 0 (0) | 0 (0) | |
| II | 3 (3.9) | 0 (0) | |
| III | 33 (43.4) | 8 (13.3) | |
| IV | 40 (52.6) | 52 (86.7) | |
| Dysmenorrhea by NRS | 4.46±2.86 | 5.37±2.50 | 0.055 |
| Use of oral contraceptivec) | 3 (3.9) | 0 (0) | 0.255 |
| Total size of endometriomas (cm) | 5.99±2.36 | 9.42±3.28 | <0.001 |
| Serum AMH | 4.09±2.63 | 4.13±2.66 | 0.918 |
| Menstrual phase of AMHd) | 0.863 | ||
| In follicular phase | 34 (44.7) | 28 (46.7) | |
| In luteal phase | 42 (55.3) | 32 (53.3) | |
| Preoperative inflammatory marker | |||
| Serum CRP (mg/dL) | 0.22±0.64 | 0.32±0.83 | 0.393 |
| Serum ESR (mm/hr) | 15.33±14.99 | 21.68±17.29 | 0.024 |
| Serum CA125 (U/mL) | 81.70±138.22 | 87.57±70.77 | 0.765 |
Values are presented as number (%) or mean±standard deviation.
rASRM, revised American Society for Reproductive Medicine; NRS, numerical rating system; AMH, anti-Müllerian hormone; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; CA125, cancer antigen 125.
a) Follicular phase length=0.770×menstrual cycle length−7.685 minutes.
b) Luteal phase length=menstrual cycle length−follicular phase duration.
c) Within 6 months before AMH was measured.
d) Menstrual phase when AMH was measured.
| Variable |
Total group |
Unilateral ovarian endometrioma |
Bilateral ovarian endometriomas |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Follicular phase (n=62) |
Luteal phase (n=74) |
Follicular phase (n=34) |
Luteal phase (n=42) |
Follicular phase (n=28) |
Luteal phase (n=32) |
|||||||
| p-value | βa) | p-value | β | p-value | β | p-value | β | p-value | β | p-value | β | |
| Age (yr) | 0.494 | 0.007b) | −0.297 | 0.819 | 0.013b) | −0.366 | 0.400 | 0.008b) | −0.441 | |||
| Length of follicular phase (day) | 0.003b) | 0.283 | 0.905 | 0.182 | 0.515 | 0.845 | 0.939 | |||||
| Length of luteal phase (day) | 0.988 | 0.001b) | 0.358 | 0.875 | 0.241 | 0.845 | 0.004b) | 0.484 | ||||
| Length of menstrual period (day) | 0.988 | 0.905 | US | 0.389 | 0.035b) | 0.400 | 0.939 | |||||
| Gravidity (time) | 0.520 | 0.465 | US | 0.881 | 0.442 | 0.984 | ||||||
| Parity (time) | 0.820 | 0.382 | US | 0.881 | 0.734 | 0.830 | ||||||
| Abortion (time) | 0.287 | 0.884 | US | US | 0.252 | 0.660 | ||||||
| Body mass index (kg/m2) | 0.104 | 0.620 | 0.675 | 0.426 | 0.113 | 0.044b) | 0.314 | |||||
| Dysmenorrheac) | 0.735 | 0.115 | 0.952 | 0.017b) | 0.352 | 0.808 | 0.880 | |||||
| Total size of endometriomas (cm) | 0.755 | 0.231 | 0.779 | 0.663 | 0.895 | 0.095 | ||||||
| Serum CRP (mg/dL) | 0.383 | 0.217 | 0.346 | 0.497 | 0.206 | 0.577 | ||||||
| ESR (mm/hr) | 0.951 | 0.289 | 0.108 | 0.436 | 0.322 | 0.275 | ||||||
| Serum CA125 (U/mL) | 0.373 | 0.861 | 0.189 | 0.458 | 0.630 | 0.165 | ||||||
US, unknown significance of the statistical program results; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; CA125, cancer antigen 125.
a) Regression coefficient: a negative value indicates an inverse relationship between the clinical factor and anti-Müllerian hormone levels. The regression coefficient (β) is not shown for factors that are not significant. Statistical significance was analyzed using multivariate linear regression.
b) Statistically significant difference.
c) Scored using numerical rating system.
- 1. Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update 2006;12:685–718.ArticlePubMed
- 2. Iwase A, Osuka S, Nakamura T, Kato N, Takikawa S, Goto M, et al. Usefulness of the ultrasensitive anti-Müllerian hormone assay for predicting true ovarian reserve. Reprod Sci 2016;23:756–60.ArticlePubMedPDF
- 3. La Marca A, Sighinolfi G, Radi D, Argento C, Baraldi E, Artenisio AC, et al. Anti-Mullerian hormone (AMH) as a predictive marker in assisted reproductive technology (ART). Hum Reprod Update 2010;16:113–30.ArticlePubMed
- 4. Wunder DM, Bersinger NA, Yared M, Kretschmer R, Birkhäuser MH. Statistically significant changes of antimüllerian hormone and inhibin levels during the physiologic menstrual cycle in reproductive age women. Fertil Steril 2008;89:927–33.ArticlePubMed
- 5. Lambert-Messerlian G, Plante B, Eklund EE, Raker C, Moore RG. Levels of antimüllerian hormone in serum during the normal menstrual cycle. Fertil Steril 2016;105:208–13.ArticlePubMed
- 6. Pankhurst MW, Chong YH. Variation in circulating antimüllerian hormone precursor during the periovulatory and acute postovulatory phases of the human ovarian cycle. Fertil Steril 2016;106:1238–43.ArticlePubMed
- 7. Muzii L, Di Tucci C, Di Feliciantonio M, Galati G, Di Donato V, Musella A, et al. Antimüllerian hormone is reduced in the presence of ovarian endometriomas: a systematic review and meta-analysis. Fertil Steril 2018;110:932–40.ArticlePubMed
- 8. Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril 2012;98:511–9.ArticlePubMed
- 9. Hwu YM, Wu FS, Li SH, Sun FJ, Lin MH, Lee RK. The impact of endometrioma and laparoscopic cystectomy on serum anti-Müllerian hormone levels. Reprod Biol Endocrinol 2011;9:80.ArticlePubMedPMCPDF
- 10. Yoon H, Lee H, Kim S, Joo J. The relationship of ovarian endometrioma and its size to the preoperative serum anti-Mullerian hormone level. Ginekol Pol 2020;91:313–9.ArticlePubMed
- 11. Karadağ C, Yoldemir T, Demircan Karadağ S, Turgut A. The effects of endometrioma size and bilaterality on ovarian reserve. J Obstet Gynaecol 2020;40:531–6.ArticlePubMed
- 12. McIntosh JE, Matthews CD, Crocker JM, Broom TJ, Cox LW. Predicting the luteinizing hormone surge: relationship between the duration of the follicular and luteal phases and the length of the human menstrual cycle. Fertil Steril 1980;34:125–30.ArticlePubMed
- 13. Revised American Fertility Society classification of endometriosis: 1985. Fertil Steril 1985;43:351–2.ArticlePubMed
- 14. Şenateş E, Çolak Y, Erdem ED, Yeşil A, Coşkunpınar E, Şahin Ö, et al. Serum anti-Müllerian hormone levels are lower in reproductive-age women with Crohn's disease compared to healthy control women. J Crohns Colitis 2013;7:e29–34.ArticlePubMed
- 15. Baek JC, Jo JY, Lee SM, Cho IA, Shin JK, Lee SA, et al. Differences in 25-hydroxy vitamin D and vitamin D-binding protein concentrations according to the severity of endometriosis. Clin Exp Reprod Med 2019;46:125–31.ArticlePubMedPMCPDF
- 16. Wu MH, Hsiao KY, Tsai SJ. Endometriosis and possible inflammation markers. Gynecol Minim Invasive Ther 2015;4:61–7.Article
- 17. Kitajima M, Matsumoto K, Murakami N, Harada A, Kitajima Y, Masuzaki H, et al. Ovarian reserve after three-step laparoscopic surgery for endometriomas utilizing dienogest: a pilot study. Reprod Med Biol 2020;19:425–31.ArticlePubMedPMCPDF
- 18. Steiner AZ, Stanczyk FZ, Patel S, Edelman A. Antimullerian hormone and obesity: insights in oral contraceptive users. Contraception 2010;81:245–8.ArticlePubMed
- 19. La Marca A, Sighinolfi G, Giulini S, Traglia M, Argento C, Sala C, et al. Normal serum concentrations of anti-Müllerian hormone in women with regular menstrual cycles. Reprod Biomed Online 2010;21:463–9.ArticlePubMed
References
Figure & Data
References
Citations

- The Relationship Between Serum Anti-Müllerian Hormone and Basal Antral Follicle Count in Infertile Women Under 35 Years: An Assessment of Ovarian Reserve
Ummey Nazmin Islam, Anwara Begum, Fatema Rahman, Md. Ahsanul Haq, Santosh Kumar, Kona Chowdhury, Susmita Sinha, Mainul Haque, Rahnuma Ahmad
Cureus.2023;[Epub] CrossRef

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite