PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 40(Suppl); 2023 > Article
-
Original article
Incidence and risk factors of deep vein thrombosis and pulmonary thromboembolism after spinal cord disease at a rehabilitation unit: a retrospective study -
Yoonhee Kim
 , Minjae Jeong
, Minjae Jeong , Myung Woo Park
, Myung Woo Park , Hyun Iee Shin
, Hyun Iee Shin , Byung Chan Lee
, Byung Chan Lee , Du Hwan Kim
, Du Hwan Kim
-
Journal of Yeungnam Medical Science 2023;40(Suppl):S56-S64.
DOI: https://doi.org/10.12701/jyms.2023.00689
Published online: September 20, 2023
Department of Physical and Rehabilitation Medicine, Chung-Ang University Hospital, Chung-Ang University College of Medicine, Seoul, Korea
- Corresponding author: Du Hwan Kim, MD, PhD Department of Physical and Rehabilitation Medicine, Chung-Ang University Hospital, Chung-Ang University College of Medicine, 102 Heukseok-ro, Dongjak-gu, Seoul 06973, Korea Tel: +82-2-6299-1865 • Fax: +82-2-6299-1886 • E-mail: ri-pheonix@hanmail.net
Copyright © 2023 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
-
Background
- Deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE) are major complications of spinal cord disease. However, studies of their incidence in Korean patients are limited. Thus, this study investigated the incidence and risk factors of DVT and PTE in Korean patients with spinal cord disease.
-
Methods
- We retrospectively analyzed the medical records of 271 patients with spinal cord disease who were admitted to a rehabilitation unit within 3 months of disease onset at a tertiary hospital. The presence of DVT and PTE was mainly determined using Doppler ultrasonography and chest embolism computed tomography. Risk factor analysis included variables such as sex, age, obesity, completeness of motor paralysis, neurological level of injury, cause of injury, lower extremity fracture, active cancer, and functional ambulation category (FAC) score.
-
Results
- The incidences of DVT and PTE in the patients with spinal cord disease were both 6.3%. Risk factor analysis revealed that age of ≥65 years (p=0.031) and FAC score of ≤1 (p=0.023) were significantly associated with DVT development. Traumatic cause of injury (p=0.028) and DVT (p<0.001) were significant risk factors of PTE.
-
Conclusion
- Patients with spinal cord disease developed DVT and PTE within 3 months of disease onset with incidence rates of 6.3% and 6.3%, respectively. Age of ≥65 years and an FAC of score ≤1 were risk factors for DVT. Traumatic cause of injury and DVT were risk factors for PTE. However, given the inconsistent results of previous studies, the risk factors for DVT and PTE remain inconclusive. Therefore, early screening for DVT and PTE should be performed in patients with acute-to-subacute spinal cord disease regardless of the presence or absence of these risk factors.
- Neurological deficits caused by spinal cord disease can result in mobility impairment in affected patients. The etiologies of spinal cord disorders include vascular diseases, diseases of the spinal column, trauma, developmental abnormalities, inflammatory diseases, metabolic and nutritional myelopathies, and tumors [1]. In terms of spinal cord injury (SCI), in reference to a national population-based study in South Korea [2], 12,137 patients were identified with acute SCI between 2007 and 2017. The age-adjusted average incidence of acute SCI was 26.4±0.9 persons per million persons and the annual percentage change for age-adjusted average incidence has increased by 2% for both men and women. Therefore, because the incidence of SCI has expanded in Korea, the incidence of spinal cord disease is also expected to increase.
- After acute care, patients with spinal cord disease and neurological deficits are usually referred to a rehabilitation ward. In the acute-to-subacute stage, patients may experience various complications. Among them, deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE) are major complications [3-7]. Patients with spinal cord disease can develop impaired hemodynamics or venous stasis due to reduced ambulation and are also likely to be in a hypercoagulable state, fulfilling Virchow’s triad [8-14]. DVT and PTE are significant causes of morbidity and mortality in patients with spinal cord disease, and PTE is the third leading cause of death in patients with SCI [15-18]. Therefore, both the prevention and recognition of DVT and PTE in patients with spinal cord disease are important.
- However, the reported incidences of DVT and PTE in patients with spinal cord disease have varied [5,19-23]. The incidence rates for DVT have ranged from 2% to 100% in patients with SCI, depending on the study population and diagnostic modality [5,19-22]. In addition, previous studies have described an incidence range for PTE of 0.4% to 6.0% [5,23,24].
- Here, we evaluated the incidence and risk factors of DVT and PTE in patients with spinal cord disease during the acute-to-subacute disease period.
Introduction
- Ethic statement: This study was approved by the Institutional Review Board (IRB) of Chung-Ang University Hospital (IRB No: 2208-028-19434), and the requirement for informed consent was waived due to its retrospective nature.
- 1. Study design and patients
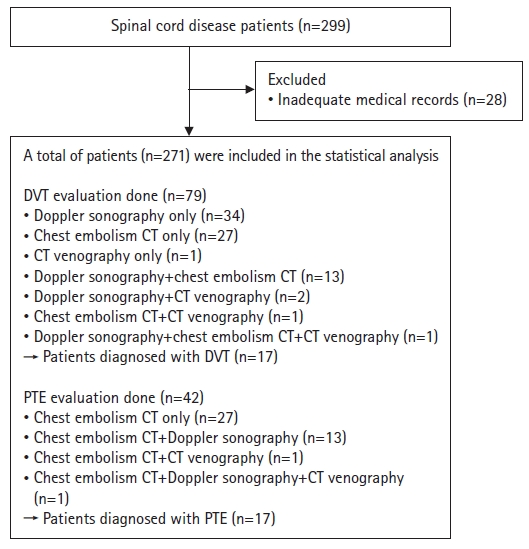
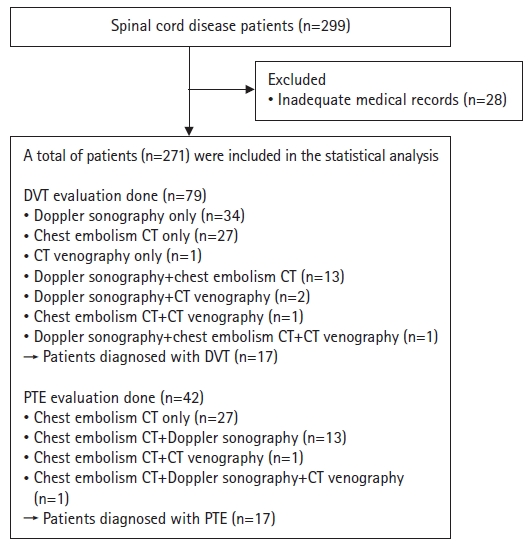
- We retrospectively reviewed the medical records of patients diagnosed with spinal cord disease between January 2012 and January 2022. The inclusion criteria were as follows: (1) diagnosis of spinal cord disease by a neurologist, neurosurgeon, or physiatrist; (2) neurologic deficits; and (3) referral to a rehabilitation ward within 3 months of disease onset. In this study, we define spinal cord disease as a condition comprising various disorders affecting the spinal cord and its surrounding structures, including traumatic SCI, degenerative spondylotic myelopathy, inflammatory spinal cord diseases, metabolic myelopathy, vascular diseases, and spinal cord tumors. Using this broad definition, we encompassed a diverse range of conditions that could affect the spinal cord. The exclusion criterion was an inadequate medical record. Initially, the medical records of 299 patients who were admitted to the rehabilitation unit or were transferred after acute surgical or medical interventions in the orthopedics, neurosurgery, or neurology departments within less than 3 months of disease onset in one tertiary referral hospital were reviewed. Patients with inadequate medical charts (n=28) were excluded. In total, 271 patients were included in this study. Among them, 79 patients underwent DVT evaluation and 42 underwent PTE evaluation (Fig. 1). Diagnostic imaging depended on the physician’s clinical decision. Patients who underwent assessment for the diagnosis of DVT or PTE were mostly those with elevated serum D-dimer concentrations in their laboratory tests, suggesting thrombosis, and/or clinical symptoms suggestive of DVT or PTE, such as leg edema, hypoxia, chest discomfort, or hypotension. Patients who did not undergo imaging evaluations for the diagnosis of DVT or PTE because of the absence of any notable events were assumed to not have DVT or PTE.
- Data were collected, including patient demographics such as age, sex, and body mass index (BMI); results of diagnostic tests for DVT or PTE; Doppler ultrasonography of the extremities; chest embolism computed tomography (CT); CT venography; any accompanying symptoms; laboratory findings (C-reactive protein [CRP], D-dimer); American Spinal Injury Association Impairment Scale (AIS); neurological level of injury (NLI); cause of injury (traumatic or nontraumatic, metastatic cord lesion or not); active cancer (cancer diagnosed within the previous 6 months); lower extremity fracture; and functional ambulation category (FAC) score.
- The anatomical locations of DVT and PTE in positive diagnostic modalities were also collected. AIS A and B categories were defined as complete motor paralysis. The NLIs were classified as cervical, thoracic, lumbar, and sacral and were further organized into subgroups: cervical as tetraplegia, and thoracic/lumbar/sacral as paraplegia. The FAC scores were categorized as ≤1 (low score) or ≥2 (high score) based on an individual's ability to ambulate independently.
- 2. Outcome measurements
- Doppler ultrasonography, chest embolism CT, and CT venography were performed to evaluate the DVT. Well-trained radiologists assessed the Doppler ultrasonography of the extremities. The deep vein system of both lower extremities was examined from the external iliac vein to the distal calf veins. Findings compatible with DVT on Doppler ultrasonography were the presence of visible echogenic material in the inner lumen, incompressible vessels with loss of phasic pattern, absence of spontaneous color mapping, and spontaneous flow via Doppler velocity. Because the protocol for chest embolism CT in our hospital covered veins in the lower extremities, it was used to diagnose DVT in the lower extremities. Chest embolism CT exploration for DVT was considered positive based on the presence of an intraluminal filling defect in the veins of the lower extremities. The deep veins in the lower extremities include the iliac, femoral, popliteal, tibial, peroneal, and soleal veins. DVTs found in the popliteal vein or more proximally were classified as proximal DVTs, whereas those in the calf veins were regarded as distal DVTs. DVT was diagnosed on CT venography when a venous filling defect was noted.
- Chest embolism CT was used to diagnose PTE. CT evaluation was considered positive for PTE based on the presence of intraluminal filling defects in the pulmonary arteries or at least partial obstruction. The pulmonary arteries were anatomically classified as main, lobar, interlobar, segmental, and subsegmental. The location of multifocal PTEs was defined by the largest artery; with multiple PTEs, the PTE located in the largest artery was considered the patient's PTE site.
- Risk factor analysis was performed for the following variables: age (≥65 years vs. <65 years), sex, obesity (BMI, ≥25 kg/m2), completeness of motor paralysis, NLI (paraplegia or tetraplegia), cause of injury (traumatic or nontraumatic, and metastatic cord lesion or not), lower extremity fracture, presence of active cancer, and low/high FAC score.
- 3. Statistical analysis
- Fisher exact and chi-square tests were used to determine the associations among different variables and the presence of PTE and DVT. These variables included sex (male vs. female), age (≥65 years vs. <65 years), obesity (BMI, ≥25 kg/m2 vs. <25 kg/m2), completeness of motor paralysis (AIS A and B vs. others), NLI (paraplegia vs. tetraplegia), cause of injury (traumatic vs. nontraumatic, and metastatic cord lesion vs. no metastatic cord lesion), presence of active cancer, lower extremity fracture, and FAC score (low vs. high). Multivariate logistic regression analysis was performed to verify the presence of independent predictive factors.
- Student t-test was used to compare the average serum CRP and D-dimer levels between patients with and without DVT or PTE. The effect sizes are reported as odds ratios (ORs) with 95% confidence intervals (95% CIs). All statistical analyses were performed using IBM SPSS ver. 19.0 (IBM Corp., Armonk, NY, USA). A p-value of <0.05 was regarded as statistically significant.
Methods
- 1. Patient demographics
- In total, 271 patients were enrolled in this study (Table 1). There were 177 male (65.3%) and 94 female patients (34.7%) with an average age of 62.7±15.9 years (mean±standard deviation). Seventy-four patients (27.3%) were obese, and 197 (72.7%) were not. Thirty-three patients (12.2%) had complete motor paralysis, and 238 (87.8%) had incomplete motor paralysis. Regarding NLI, 146 patients (53.9%) had tetraplegia, and 125 (46.1%) had paraplegia. Trauma was the cause of spinal cord disease in 142 patients (52.4%), whereas 129 patients (47.6%) had nontraumatic causes. Metastatic spinal cord lesions were discovered in three patients (1.1%). Nine patients (3.3%) had active cancer and 11 (4.1%) had lower extremity fractures. A low FAC score was observed in 152 patients (56.1%), and a high FAC score was observed in 119 patients (43.9%).
- 2. Incidence and clinical findings of deep vein thrombosis and pulmonary thromboembolism
- Among the 271 patients, 79 underwent DVT evaluation using venous duplex Doppler ultrasound, chest embolism CT, or CT venography, and 42 patients were assessed for PTE using chest embolism CT (Fig. 1). Among the involved patients (n=271), DVT was diagnosed in 17 (6.3%) and PTE was diagnosed in 17 (6.3%).
- Among the patients evaluated for DVT (n=79), 73 did not receive any prophylaxis with anticoagulants and six patients did. Of the latter six patients, one received prophylaxis for DVT, and the other five received an anticoagulant because of underlying atrial fibrillation. Three of the six patients who received prophylaxis and 14 of the 73 patients who did not receive prophylaxis were diagnosed with DVT. Among the patients who were evaluated for PTE (n=42), 39 did not receive any prophylaxis with anticoagulants and three patients did. Of the latter three patients, one received prophylaxis for PTE, and the other two received an anticoagulant because of underlying atrial fibrillation. Two of the three patients who received prophylaxis and 15 of the 39 patients who did not receive prophylaxis were diagnosed with PTE.
- Among the patients diagnosed with DVT (n=17), 12 had symptomatic DVT, such as leg edema and PTE-related chest pain or hypoxia, and four had asymptomatic DVT. Owing to the lack of records, whether one patient was symptomatic is unknown. Among the patients diagnosed with PTE (n=17), 12 had symptomatic PTE, including hypoxia, chest discomfort, hypotension, and DVT-related leg edema, and five had asymptomatic PTE.
- Regarding the anatomical location of the lesions, distal DVT was present in five of 17 patients (29.4%) and proximal DVT was observed in 12 of 17 patients (70.6%). Regarding the distribution of PTE in the arterial segments, when a patient had multiple PTEs, we considered the PTE in the largest artery to be the patient’s PTE site. Two, five, seven, and three patients had a PTE in the main pulmonary, lobar pulmonary, segmental, and subsegmental artery, respectively.
- 3. Comparison of serum C-reactive protein and D-dimer concentrations
- We compared mean serum CRP and D-dimer levels between patients with and without DVT or PTE. In the case of DVT, there was no statistically significant difference in the mean serum CRP concentrations between the patients with DVT (56.34 mg/L) and those without DVT (19.79 mg/L; p=0.06; range, 0–5.0 mg/L). However, in patients with DVT, a statistically significant elevation in mean serum D-dimer concentration (5.71 µg/mL) was observed compared to those in patients without DVT (2.66 µg/mL; p<0.001; range, 0–0.5 µg/mL).
- Similarly, there was no statistically significant difference in the mean serum CRP concentration between patients with PTE (40.11 mg/L) and those without PTE (20.74 mg/L; p=0.08; range, 0–5.0 mg/L). However, there was a statistically significant difference in the mean serum D-dimer concentration between patients with PTE (6.39 µg/mL) and those without PTE (2.53 µg/mL; p=0.001; range, 0–0.5 µg/mL).
- 4. Risk factor analysis of deep vein thrombosis and pulmonary thromboembolism development
- Sex (p=0.27), obesity (p>0.99), completeness of motor paralysis (p=0.14), NLI (p=0.94), traumatic cause (p=0.96), metastatic cord lesions (p=0.18), active cancer (p=0.45), and lower extremity fracture (p>0.99) were not significantly associated with the occurrence of DVT. Univariate analysis revealed that age of ≥65 years (p=0.011) and low FAC score (p=0.006) were significantly associated with the development of DVT (Table 2). Multivariate analysis confirmed that age of ≥65 years (OR, 4.091; 95% CI, 1.135–14.744; p=0.031) and low FAC score (OR, 5.761; 95% CI, 1.280–25.925; p=0.023) were significant independent risk factors (Table 3).
- Factors such as sex (p=0.56), age (p=0.58), obesity (p>0.99), NLI (p=0.67), metastatic cord lesions (p=0.18), active cancer (p=0.45), and lower extremity fractures (p=0.52) were not significantly correlated with PTE (Table 2). Univariate analysis revealed that completeness of motor paralysis (p=0.01), traumatic cause of injury (p=0.04), low FAC score (p=0.024), and DVT (p<0.001) were significantly associated with the incidence of PTE. In the multivariate analysis, traumatic cause of injury (OR, 4.742; 95% CI, 1.188–18.939; p=0.028) and DVT (OR, 44.961; 95% CI, 12.011–168.308; p<0.001) were significant independent risk factors for PTE (Table 3).
Results
- We evaluated the incidence and risk factors of DVT and PTE in patients with spinal cord disease in an acute-to-subacute rehabilitation unit in Korea. The incidences of DVT and PTE were 6.3% and 6.3%, respectively. In the context of DVT development, age of ≥65 years and low FAC scores were identified as significant independent risk factors. Traumatic causes of injury and DVT were established as significant independent risk factors for PTE.
- In this study, the incidence of DVT in spinal cord disease was 6.3%, which was lower than that reported in previous studies on DVT incidence in patients with SCI [22,25-28]. One retrospective study of Korean patients with traumatic/nontraumatic SCI determined that DVT was detected in 51 of 185 patients (27.6%) at the time of initial presentation to a rehabilitation unit, based on Doppler ultrasonography findings [27]. In a retrospective study of 52 Japanese patients with acute cervical SCI, 11 of 52 patients (21.2%) were diagnosed with DVT by Doppler ultrasonography [22]. In addition, a prospective study of a small number of Korean patients indicated that 16 of 37 patients (43%) with acute SCI had DVT, which was also based on Doppler ultrasonography [28]. Matsumoto et al. [26] reported that 12 of 29 Japanese patients (41.4%) with acute SCI had DVT. Aito et al. [25] reported that the incidence of DVT in patients with SCI ranged from 2% (with prophylaxis) to 26% (without prophylaxis). It is important to acknowledge the inherent limitations of our retrospective study, which may have underestimated the true incidence of DVT. Additional research is required to prospectively assess the incidence of DVT and validate the results reported herein. Furthermore, in this study, the fact that only 79 of 271 study subjects underwent diagnostic evaluation of DVT can be considered a factor that may have contributed to the lower incidence compared to that found in previous studies. As routine screening and imaging evaluations were not performed for all patients in this study, there is a possibility that asymptomatic DVT occurrence was missed. This can be regarded as a major limitation of our study; however, when the evaluations were conducted, a diagnosis of DVT was confirmed in 17 of 79 patients (21.5%), and proximal DVT was identified at a significantly higher rate of 70.6%. It can be inferred that a higher DVT incidence would have been obtained if asymptomatic DVT had been included. Therefore, it is crucial to actively conduct DVT screening in patients with spinal cord disease, considering the high incidence, morbidity, and mortality associated with this condition.
- Regarding the anatomical location of the DVT, distal DVT was present in five of 17 patients (29.4%), whereas proximal DVT was found in 12 of 17 patients (70.6%). Given that these data were based on 17 patients, interpretation of the significance is limited. However, the higher incidence of proximal DVT than distal DVT in our study could be attributed to the diagnostic tools used, such as ultrasound and CT. The smaller size of distal veins compared to that of proximal veins increases the potential for false negatives in the former. However, these findings were based on a limited sample size of 17 patients. Therefore, further research with a larger sample size is necessary to comprehensively investigate this phenomenon.
- In addition, the incidence of PTE in our study was 6.3%, which is similar to the values reported in previous studies, which ranged from 0.4% to 6.0% [5,23]. A systematic review of PTE in the subacute phase of SCI revealed a PTE incidence of 0.5% to 6.0% [5]. A retrospective study of 1,485 patients who underwent spinal surgery at a single tertiary care center revealed a PTE incidence of 0.4% after the surgery [23]. Therefore, the incidence of PTE in patients with spinal cord disease in an acute-to-subacute rehabilitation unit setting was estimated to be approximately 6%, which is consistent with the findings of previous studies.
- We compared mean serum CRP and D-dimer levels between patients with and without DVT or PTE. Although no statistically significant difference was observed in serum CRP concentrations between the group diagnosed with DVT or PTE and the group without DVT or PTE, the difference was close to significance. In contrast, a statistically significant difference was observed in serum D-dimer concentrations between the group diagnosed with DVT or PTE and that without DVT or PTE. As previously reported in studies, D-dimer demonstrates high sensitivity (more than 95%) but limited specificity in the diagnosis of DVT and PTE [29-32]. Therefore, patients with spinal cord disease should be screened for DVT and PTE when their serum D-dimer concentrations exceed the normal threshold.
- In this study, risk factor analysis demonstrated that age of ≥65 years and low FAC score were significant independent risk factors for DVT. A traumatic cause of injury and DVT were significant independent risk factors for PTE. These findings are consistent with results from previous studies, in that many previous studies on the risk factors for DVT in patients with SCI have failed to reach concordance [19,20,23,27]. A retrospective study of 1,485 cases of spinal surgery revealed that patients with a prior history of DVT or PTE, estrogen replacement therapy, discharge to a rehabilitation facility, and major depressive disorder presented a significantly higher relative risk [23]. Another study of 185 inpatients with traumatic/nontraumatic SCI found that the absence of spasticity was the only significant risk factor in the study population [27]. In a study involving 189 patients with traumatic SCI, tetraplegia and paraplegia were found to be significant risk factors [19]. In addition, a 1-week study of 151 patients with traumatic/nontraumatic SCI showed that male, age above 50 years, and comorbidities were risk factors [20].
- Despite its importance, a comprehensive understanding of the risk factors for DVT and PTE in spinal cord disease is lacking. The discordance with previous studies may have originated from the heterogeneity of the study populations, or differences in the time of diagnosis or study design. However, the incidence of DVT was clearly high in patients with spinal cord disease. Spinal cord disease itself should be considered a risk factor for DVT and subsequent PTE. In addition to the high incidence of DVT in patients with SCI in previous studies, according to a nationwide cohort prospective study conducted in Taiwan, patients with SCI have a 2.46-fold adjusted hazard ratio (aHR) for DVT and 1.57-fold aHR for PTE, compared with that of the general population [33]. These results indicate that each patient with spinal cord disease admitted to an acute-to-subacute rehabilitation unit needs a routine check-up for DVT and subsequent PTE, regardless of sex, age, NLI, pathophysiology, or functional status.
- This study had several limitations. First, it was retrospective. Larger prospective studies are needed to better understand the clinical relevance of our results. Second, assessments of DVT and PTE were not performed routinely; therefore, the number of patients who underwent evaluation for DVT or PTE was limited. Furthermore, considering that the patients who underwent evaluation for DVT or PTE were mostly those with high serum D-dimer concentrations or clinical suspicion based on symptoms such as leg edema or respiratory symptoms, it is plausible that asymptomatic individuals with DVT and PTE might have been overlooked. Consequently, it is reasonable to suggest that the incidences of DVT and PTE may have been significantly underestimated in this study. Finally, the diagnostic methods for DVT varied. Further studies are necessary using standardized diagnostic tools for DVT.
- In conclusion, the incidences of DVT and PTE in patients with spinal cord disease admitted to a rehabilitation unit less than 3 months from disease onset were 6.3% and 6.3%, respectively. Age of ≥65 years and low FAC score were established as significant risk factors for DVT in patients with spinal cord disease. In addition, traumatic causes of injury and DVT were independent risk factors for PTE in patients with spinal cord disease. Given the lack of consistent findings in previous studies, there is no conclusive evidence regarding the risk factors for DVT and PTE. However, it is evident that spinal cord disease itself is a clear risk factor for both conditions. In conclusion, patients with spinal cord disease admitted to a rehabilitation unit within 3 months of disease onset should be considered at high risk for DVT and subsequent PTE and should undergo a screening workup for both DVT and PTE.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
The study was supported by the Health Fellowship Foundation.
-
Author contributions
Conceptualization: DHK; Data curation, Investigation, Visualization: YK, MJ; Funding acquisition, Formal analysis: YK; Methodology: DHK; Supervision: MWP, HIS, BCL, DHK; Writing-original draft: YK; Writing-review & editing: BCL, DHK.
Notes
| Parameter | Data |
|---|---|
| No. of patients | 271 |
| Sex | |
| Male:female | 177 (65.3):94 (34.7) |
| Age (yr) | 62.7±15.9 |
| Obesity | |
| Yesa) | 74 (27.3) |
| No | 197 (72.7) |
| Motor paralysis completeness | |
| Yes (AIS A or B) | 33 (12.2) |
| No (AIS C or D) | 238 (87.8) |
| Neurologic level of injury | |
| Cervical | 146 (53.9) |
| Thoracic | 57 (21.0) |
| Lumbar | 64 (23.6) |
| Sacral | 4 (1.5) |
| Cause of injury | |
| Traumatic | 142 (52.4) |
| Nontraumatic | 129 (47.6) |
| Metastatic spinal cord lesion | |
| Yes | 3 (1.1) |
| No | 268 (98.9) |
| Active cancer | |
| Yes | 9 (3.3) |
| No | 262 (96.7) |
| Lower extremity fracture | |
| Yes | 11 (4.1) |
| No | 260 (95.9) |
| FAC score | |
| Low (≤1) | 152 (56.1) |
| High (≥2) | 119 (43.9) |
| Parameter | No. of patients |
DVT |
PTE |
||||
|---|---|---|---|---|---|---|---|
| No. (%) | p-value | OR (95% CI) | No. (%) | p-value | OR (95% CI) | ||
| Sex | |||||||
| Male | 177 | 9 (5.1) | 0.27 | 1.736 (0.647–4.660) | 10 (5.6) | 0.56 | 1.344 (0.494–3.653) |
| Femalea) | 94 | 8 (8.5) | 7 (7.4) | ||||
| Age (yr) | |||||||
| ≥65a) | 142 | 14 (9.9) | 0.011* | 4.594 (1.289–16.374) | 10 (7.0) | 0.58 | 1.320 (0.487–3.577) |
| <65 | 129 | 3 (2.3) | 7 (5.4) | ||||
| Obesity | |||||||
| Yesa) | 74 | 4 (5.4) | >0.99 | 0.809 (0.255–2.564) | 4 (5.4) | >0.99 | 0.809 (0.255–2.564) |
| No | 197 | 13 (6.6) | 13 (6.6) | ||||
| Motor paralysis completeness | |||||||
| Yesa) | 33 | 4 (12.1) | 0.14 | 2.387 (0.730–7.812) | 6 (18.2) | 0.01* | 4.586 (1.570 –13.393) |
| No | 238 | 13 (5.5) | 11 (4.6) | ||||
| Neurologic level of injury | |||||||
| Tetraplegia (cervical)a) | 146 | 9 (6.2) | 0.94 | 0.961 (0.359–2.570) | 10 (6.8) | 0.67 | 1.239 (0.457–3.359) |
| Paraplegia (others) | 125 | 8 (6.4) | 7 (5.6) | ||||
| Cause of injury | |||||||
| Traumatica) | 142 | 9 (6.3) | 0.96 | 1.023 (0.383–2.737) | 13 (9.2) | 0.04* | 3.149 (1.000–9.919) |
| Nontraumatic | 129 | 8 (6.2) | 4 (3.1) | ||||
| Metastatic spinal cord lesion | |||||||
| Yesa) | 3 | 1 (33.3) | 0.18 | 7.875 (0.677–91.538) | 1 (33.3) | 0.18 | 7.875 (0.677–91.538) |
| No | 268 | 16 (6.0) | 16 (6.0) | ||||
| Active cancer | |||||||
| Yesa) | 9 | 1 (11.1) | 0.45 | 1.922 (0.226–16.326) | 1 (33.3) | 0.45 | 1.922 (0.226–16.326) |
| No | 262 | 16 (6.1) | 16 (6.0) | ||||
| Lower extremity fracture | |||||||
| Yesa) | 11 | 0 (0) | >0.99 | 0.935 (0.905–0.965) | 1 (33.3) | 0.52 | 1.525 (0.184–12.666) |
| No | 260 | 17 (6.5) | 16 (6.0) | ||||
| FAC score | |||||||
| Low (≤1)a) | 152 | 15 (9.9) | 0.006* | 6.405 (1.435–28.588) | 14 (9.2) | 0.024* | 3.923 (1.100–13.984) |
| High (≥2) | 119 | 2 (1.7) | 3 (2.5) | ||||
| DVT | |||||||
| Yesa) | 17 | 9 (52.9) | <0.001* | 35.594 (10.583–113.078) | |||
| No | 254 | 8 (3.1) | |||||
| Parameter | β coefficient | SE β | p-value | OR (95% CI) |
|---|---|---|---|---|
| DVT | ||||
| Age, ≥65 yr | 1.409 | 0.654 | 0.031* | 4.091 (1.135–14.744) |
| Low FAC score (≤1) | 1.751 | 0.767 | 0.023* | 5.761 (1.280–25.925) |
| PTE | ||||
| Traumatic cause of injury | 1.557 | 0.706 | 0.028* | 4.742 (1.188–18.939) |
| DVT | 3.806 | 0.673 | <0.001* | 44.961 (12.011–168.308) |
- 1. de Girolami U, Bale TA. Spinal cord. Handb Clin Neurol 2017;145:405–25.ArticlePubMed
- 2. Choi SH, Sung CH, Heo DR, Jeong SY, Kang CN. Incidence of acute spinal cord injury and associated complications of methylprednisolone therapy: a national population-based study in South Korea. Spinal Cord 2020;58:232–7.ArticlePubMedPDF
- 3. Godat LN, Kobayashi L, Chang DC, Coimbra R. Can we ever stop worrying about venous thromboembolism after trauma? J Trauma Acute Care Surg 2015;78:475–81.ArticlePubMed
- 4. Agarwal NK, Mathur N. Deep vein thrombosis in acute spinal cord injury. Spinal Cord 2009;47:769–72.ArticlePubMedPDF
- 5. Alabed S, Belci M, Van Middendorp JJ, Al Halabi A, Meagher TM. Thromboembolism in the sub-acute phase of spinal cord injury: a systematic review of the literature. Asian Spine J 2016;10:972–81.ArticlePubMedPMCPDF
- 6. Dhall SS, Hadley M, Aarabi B, Gelb DE, Hurlbert RJ, Rozzelle CJ, et al. Deep venous thrombosis and thromboembolism in patients with cervical spinal cord injuries. Neurosurgery 2013;72(Suppl 2):244–54.ArticlePubMedPDF
- 7. Paciaroni M, Ageno W, Agnelli G. Prevention of venous thromboembolism after acute spinal cord injury with low-dose heparin or low-molecular-weight heparin. Thromb Haemost 2008;99:978–80.ArticlePubMed
- 8. Merli GJ, Herbison GJ, Ditunno JF, Weitz HH, Henzes JH, Park CH, et al. Deep vein thrombosis: prophylaxis in acute spinal cord injured patients. Arch Phys Med Rehabil 1988;69:661–4.PubMed
- 9. Golan DE, Tashjian AH, Armstrong EJ. Principles of pharmacology: the pathophysiologic basis of drug therapy. 3rd ed. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011.
- 10. Furlan JC, Fehlings MG. Role of screening tests for deep venous thrombosis in asymptomatic adults with acute spinal cord injury: an evidence-based analysis. Spine (Phila Pa 1976) 2007;32:1908–16.ArticlePubMed
- 11. Anderson FA, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003;107(23 Suppl 1):I9–16.ArticlePubMed
- 12. Merli GJ, Crabbe S, Paluzzi RG, Fritz D. Etiology, incidence, and prevention of deep vein thrombosis in acute spinal cord injury. Arch Phys Med Rehabil 1993;74:1199–205.ArticlePubMed
- 13. Miranda AR, Hassouna HI. Mechanisms of thrombosis in spinal cord injury. Hematol Oncol Clin North Am 2000;14:401–16.ArticlePubMed
- 14. Yao JS. Deep vein thrombosis in spinal cord-injured patients. Evaluation and assessment. Chest 1992;102(6 Suppl):645S–648S.ArticlePubMed
- 15. Jackson AB, Groomes TE. Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehabil 1994;75:270–5.ArticlePubMed
- 16. DeVivo MJ, Kartus PL, Stover SL, Rutt RD, Fine PR. Cause of death for patients with spinal cord injuries. Arch Intern Med 1989;149:1761–6.ArticlePubMed
- 17. Green D, Hartwig D, Chen D, Soltysik RC, Yarnold PR. Spinal cord injury risk assessment for thromboembolism (SPIRATE Study). Am J Phys Med Rehabil 2003;82:950–6.ArticlePubMed
- 18. Waring WP, Karunas RS. Acute spinal cord injuries and the incidence of clinically occurring thromboembolic disease. Paraplegia 1991;29:8–16.ArticlePubMedPDF
- 19. Hon B, Botticello A, Kirshblum S. Duplex ultrasound surveillance for deep vein thrombosis after acute traumatic spinal cord injury at rehabilitation admission. J Spinal Cord Med 2020;43:298–305.ArticlePubMed
- 20. Piran S, Schulman S. Incidence and risk factors for venous thromboembolism in patients with acute spinal cord injury: a retrospective study. Thromb Res 2016;147:97–101.ArticlePubMed
- 21. Mackiewicz-Milewska M, Cisowska-Adamiak M, Pyskir J, Świątkiewicz I. Usefulness of D-dimer and ultrasonography screening for detecting deep vein thrombosis in patients with spinal cord injury undergoing rehabilitation. J Clin Med 2021;10:689.ArticlePubMedPMC
- 22. Sugimoto Y, Ito Y, Tomioka M, Tanaka M, Hasegawa Y, Nakago K, et al. Deep venous thrombosis in patients with acute cervical spinal cord injury in a Japanese population: assessment with Doppler ultrasonography. J Orthop Sci 2009;14:374–6.ArticlePubMed
- 23. Schulte LM, O'Brien JR, Bean MC, Pierce TP, Yu WD, Meals C. Deep vein thrombosis and pulmonary embolism after spine surgery: incidence and patient risk factors. Am J Orthop (Belle Mead NJ) 2013;42:267–70.PubMed
- 24. Teasell RW, Hsieh JT, Aubut JA, Eng JJ, Krassioukov A, Tu L, et al. Venous thromboembolism after spinal cord injury. Arch Phys Med Rehabil 2009;90:232–45.ArticlePubMedPMC
- 25. Aito S, Pieri A, D'Andrea M, Marcelli F, Cominelli E. Primary prevention of deep venous thrombosis and pulmonary embolism in acute spinal cord injured patients. Spinal Cord 2002;40:300–3.ArticlePubMedPDF
- 26. Matsumoto S, Suda K, Iimoto S, Yasui K, Komatsu M, Ushiku C, et al. Prospective study of deep vein thrombosis in patients with spinal cord injury not receiving anticoagulant therapy. Spinal Cord 2015;53:306–9.ArticlePubMedPDF
- 27. Do JG, Kim du H, Sung DH. Incidence of deep vein thrombosis after spinal cord injury in Korean patients at acute rehabilitation unit. J Korean Med Sci 2013;28:1382–7.ArticlePubMedPMCPDF
- 28. Chung SB, Lee SH, Kim ES, Eoh W. Incidence of deep vein thrombosis after spinal cord injury: a prospective study in 37 consecutive patients with traumatic or nontraumatic spinal cord injury treated by mechanical prophylaxis. J Trauma 2011;71:867–71.ArticlePubMed
- 29. Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med 2003;349:1227–35.ArticlePubMed
- 30. Masuda M, Ueta T, Shiba K, Iwamoto Y. D-dimer screening for deep venous thrombosis in traumatic cervical spinal injuries. Spine J 2015;15:2338–44.ArticlePubMed
- 31. Roussi J, Bentolila S, Boudaoud L, Casadevall N, Vallée C, Carlier R, et al. Contribution of D-Dimer determination in the exclusion of deep venous thrombosis in spinal cord injury patients. Spinal Cord 1999;37:548–52.ArticlePubMedPDF
- 32. Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet 2012;379:1835–46.ArticlePubMed
- 33. Chung WS, Lin CL, Chang SN, Chung HA, Sung FC, Kao CH. Increased risk of deep vein thrombosis and pulmonary thromboembolism in patients with spinal cord injury: a nationwide cohort prospective study. Thromb Res 2014;133:579–84.ArticlePubMed
References
Figure & Data
References
Citations

- Coagulation parameters correlate to venous thromboembolism occurrence during the perioperative period in patients with spinal fractures
Yong Jiao, Xiaohong Mu
Journal of Orthopaedic Surgery and Research.2023;[Epub] CrossRef

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite