PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 38(4); 2021 > Article
-
Case report
Pembrolizumab-related autoimmune hemolytic anemia in a patient with metastatic lung adenocarcinoma: a case report -
Dong Won Baek
 , Yee Soo Chae
, Yee Soo Chae
-
Yeungnam University Journal of Medicine 2021;38(4):366-370.
DOI: https://doi.org/10.12701/yujm.2021.00899
Published online: March 23, 2021
Department of Hematology/Oncology, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, Daegu, Korea
- Corresponding author: Yee Soo Chae, MD, PhD Department of Hematology/Oncology, School of Medicine, Kyungpook National University, Kyungpook National University Chilgok Hospital, 807 Hoguk-ro, Buk-gu, Daegu 41404, Korea Tel: +82-53-200-2623 Fax: +82-53-200-2029 E-mail: yschae@knu.ac.kr
Copyright © 2021 Yeungnam University College of Medicine
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 5,166 Views
- 120 Download
- 5 Crossref
Abstract
- Immune checkpoint inhibitors (ICIs) have become the main drugs for programmed cell death receptor-1 or ligand-1 expressing non-small cell lung cancer (NSCLC) combined with conventional chemotherapy. ICIs are generally more tolerable than cytotoxic chemotherapies in terms of toxicity, and ICI-related adverse events are mild and manageable. However, these drugs may lead to unexpected severe adverse events such as immune-related hematologic toxicities, which could be life-threatening. Here, a rare case of a pembrolizumab-related adverse event in a patient with NSCLC who showed early-onset hemolytic anemia and recovered by high-dose steroid and a series of plasma exchanges is reported.
- Efforts to turn on the immune system against cancers have led to the development of immune checkpoint inhibitors (ICIs) targeting programmed cell death receptor-1 or ligand-1 (PD-1/PD-L1) [1]. Since the first checkpoint molecular inhibitor ipilimumab was approved by the U.S. Food and Drug Administration (FDA), anti-PD-1/PD-L1 therapies are widely used in various cancers to improve survival outcomes, particularly in metastatic non-small cell lung cancer (NSCLC) and melanoma [2].
- Although tumor cells escape immune attack through various complementary mechanisms of immunosuppression, ICIs targeting immunosuppressive molecules, such as cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and PD-1/PD-L1, can reactivate cytotoxic T cells to kill malignant cells [3]. Meanwhile, ICIs break the balance of the immune system, which may lead to the development of immune-related adverse events (IRAEs) in some patients. Generally, IRAEs can show variable autoimmune manifestations involving the skin, gastrointestinal tract, endocrine system, lung, joints, and many other organs [4]. Rarely, hematologic manifestations, such as cytopenias, have also been reported in patients with solid tumors and lymphomas during ICI treatment [5]. Most IRAEs are manageable by suppressing lymphocyte activation with steroids. However, some severe cases require changes in the treatment strategy [6].
- Currently, pembrolizumab has become the first-line standard regimen for metastatic NSCLC with positive PD-L1 expression and negative actionable mutations for targeted therapies [7]. However, clinicians are faced with unexpected IRAEs and need more experience to overcome IRAEs. This paper reports a rare case of autoimmune hemolytic anemia (AIHA) in a patient with NSCLC receiving a pembrolizumab-containing regimen for metastatic lung cancer.
Introduction
- A 70-year-old male newly diagnosed with metastatic NSCLC visited our oncology department in September 2020. The biopsied lung tissue revealed a poorly differentiated adenocarcinoma. Immunohistochemistry analysis showed overexpression of PD-L1 (100% by 22C3 pharmDx assay) and no alterations for targeted therapy including epidermal growth factor receptor, anaplastic lymphoma kinase, or c-ros oncogene 1. The patient’s general condition was good with an Eastern Cooperative Oncology Group performance status of 1, and he had no history of other diseases. He received pembrolizumab plus pemetrexed and cisplatin as first-line therapy for metastatic NSCLC based on international guidelines. His baseline complete blood cell count analysis at the beginning of chemotherapy revealed a white blood cell count of 11,450/μL, hemoglobin (Hb) 13.4 g/dL, and platelet (PLT) count of 218,000/μL.
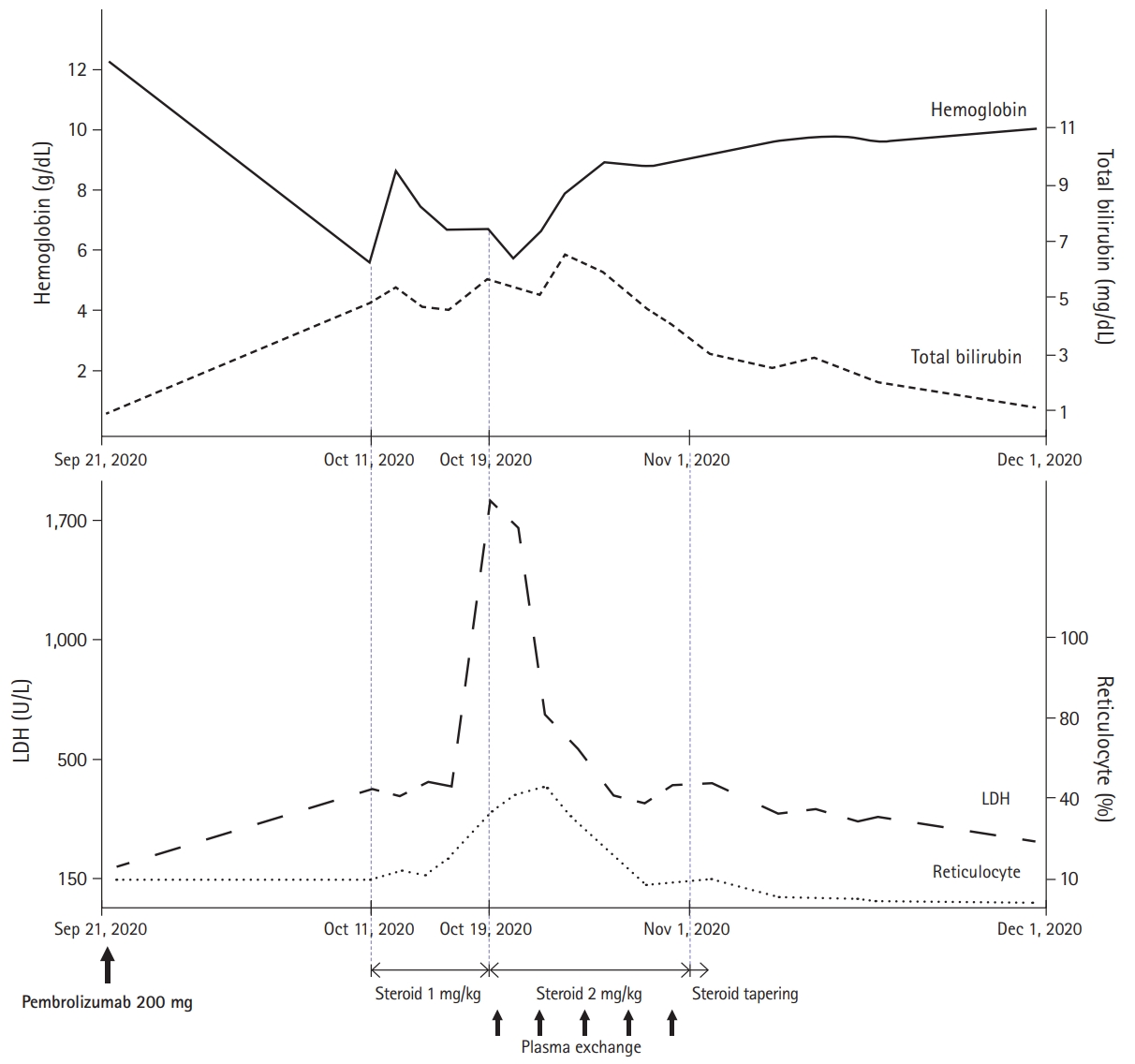
- Two weeks later, he complained of worsening dyspnea and was admitted for evaluation. Results of blood test showed Hb of 5.8 g/dL, total bilirubin of 4.16 mg/dL (direct bilirubin, 0.59 mg/dL and indirect bilirubin, 3.57 mg/dL), depletion of haptoglobin (<10 mg/dL; range, 30–200 mg/dL), and increased serum lactate dehydrogenase (LDH) (478 U/L; range, 140–271 U/L). Over 20% of spherocytes were identified in the peripheral blood smear, and the direct antiglobulin test (DAT) and cold agglutinin test were positive (Table 1). With an increased reticulocyte count, we considered his severe anemia as AIHA, a rare IRAE of ICIs. The patient was administered prednisolone (1 mg/kg). Despite using high-dose steroids over a week, his LDH continuously increased over 1,000 U/L and PLT count decreased to <100,000/μL without any sign of improvement in hemolytic anemia. Therefore, he was started on prednisolone at 2 mg/kg and underwent plasma exchange five times every other day. Features of hemolytic anemia showed significant improvement after 2 weeks of increased steroid dose and plasma exchange; therefore, the steroid dose was tapered slowly up to 10 mg/day while maintaining the normalized hemoglobin level. The patient then received subsequent conventional chemotherapy with pemetrexed plus cisplatin, and there was no recurrence of hemolysis. The overall treatment and progress are summarized in Fig. 1.
Case
- Previous studies have reported that the frequency of immune-mediated cytopenias is <0.5% in patients treated with ICIs [8]. According to the details of 68 AIHA cases identified in the FDA database, AIHA was relatively infrequent with pembrolizumab and ipilimumab compared to atezolizumab and nivolumab (Table 2) [9]. PD-1 inhibitor-associated AIHA has been reported to usually occur after two to five cycles of treatment compared with 8 to 12 weeks with CTLA-4 inhibitor therapy [10]. In the described case, the patient showed features of hemolytic anemia with positive DAT and the presence of cold agglutinin after only one cycle of pembrolizumab, including combination chemotherapy. Generally, a positive DAT is an important AIHA feature, and >50% of immune-mediated anemia show positive DAT with immunoglobulin (Ig) G or C3. Cold agglutinin disease (CAD) is another form of AIHA that accounts for 10% to 20% of AIHA. Hemolysis of CAD is primarily extravascular, which is mediated by IgM, cold agglutinin, and complements at low temperatures [11]. Although pembrolizumab-associated AIHA with cold agglutinin and DAT is rare, a similar case of metastatic lung cancer has been reported [12].
- The general recommendations for AIHA management include steroids and consideration of plasma exchange, and the guidelines on the management of ICI-related AIHA are similar. The key mechanism of IRAEs is that ICI therapies disrupt immunological homeostasis and reduce T-cell tolerance [13]. Therefore, the European Society of Medical Oncology suggests that high-dose corticosteroids and/or other immunosuppressive drugs should be considered in cooperation with a hematologist [14]. The American Society of Clinical Oncology recommends discontinuation of ICIs and commencement of prednisone (1–2 mg/kg/day) with red blood cell transfusion targeting Hb of 7 to 8 g/dL [15]. Previous studies have reported that immune-related anemia may be steroid-resistant, and 1 mg/kg of prednisone may be insufficient to resolve hemolysis [5,8]. In particular, some cases with nivolumab showed a poor response to steroids, resulting in fatal outcomes. Conversely, some cases with pembrolizumab-related AIHA were relatively not severe and responded to steroids [9,16,17]. In our experience, there was little effect with 1 mg/kg of prednisone, and the features of severe hemolytic anemia began to improve by increasing the prednisone dose to 2 mg/kg/day. Maintaining high-dose steroids over a week with plasma exchange was also important in controlling hemolysis and preventing recurrence.
- ICI therapy-associated AIHA is rare, but most cases can be life-threatening. Recently, the number of reported cases has increased with the expansion of ICIs and the number of patients exposed to them. Early recognition is important, and we hope that patients with ICI-related AIHA can receive appropriate management, including high-dose steroid and plasma exchange, through this case report. Further studies with immune cell profiling in patients with hematological IRAEs should be performed to better understand its mechanism and adequate management. In addition, trials including other ICIs with reportedly less hematological IRAEs for patients who experienced immune-related hemolytic anemia should be considered.
Discussion
-
Ethical statements
This study was approved by the Institutional Review Board (IRB) of Kyungpook National University Chilgok Hospital (IRB No: KNUCH 2021-02-007). The patient provided written informed consent for publication of clinical details.
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Author contributions
Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Visualization, Investigation, Validation: all authors; Resources, Supervision: YSC, Writing-original draft, Writing-review & editing: all authors.
Notes
| Variable | Ipilimumab | Nivolumab | Pembrolizumab | Atezolizumab |
|---|---|---|---|---|
| Case of AIHA/OAEa) | 7/12,631 (0.055) | 43/20,335 (0.211) | 13/8,917 (0.146) | 5/2,021 (0.247) |
| Age (yr)b) | 65 (32–68) | 68 (43–85) | 62 (35–82) | 67 (57–69) |
| Underlying malignancy | MM: 7 | LC: 18, MM: 17c), HL: 3c), RCC: 2, Others: 4 | MM: 7, LC: 5, Others: 1 | BC: 2, LC: 1, MM: 1, OC: 1 |
| Other reported hematological adverse event with AIHA | Agranulocytosis, bicytopenia | ITP | ITP, PRCA | - |
AIHA, autoimmune hemolytic anemia; OAE, overall adverse events; FDA, U.S. Food and Drug Administration; MM, malignant melanoma; LC, lung cancer; HL, Hodgkin lymphoma; RCC, renal cell carcinoma; BC, breast cancer; OC, ovarian cancer; ITP, immune thrombocytopenic purpura; PRCA, pure red cell aplasia.
a) Number (%).
b) Median (range).
c) One patient had both HL and MM.
- 1. Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med 2012;366:2517–9.ArticlePubMed
- 2. Dobosz P, Dzieciątkowski T. The intriguing history of cancer immunotherapy. Front Immunol 2019;10:2965.ArticlePubMedPMC
- 3. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011;480:480–9.ArticlePubMedPMC
- 4. Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, et al. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer 2016;54:139–48.ArticlePubMed
- 5. Calvo R. Hematological side effects of immune checkpoint inhibitors: the example of immune-related thrombocytopenia. Front Pharmacol 2019;10:454.ArticlePubMedPMC
- 6. Weber JS, Hodi FS, Wolchok JD, Topalian SL, Schadendorf D, Larkin J, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol 2017;35:785–92.ArticlePubMed
- 7. Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018;378:2078–92.ArticlePubMed
- 8. Delanoy N, Michot JM, Comont T, Kramkimel N, Lazarovici J, Dupont R, et al. Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: a descriptive observational study. Lancet Haematol 2019;6:e48–57.ArticlePubMed
- 9. Tanios GE, Doley PB, Munker R. Autoimmune hemolytic anemia associated with the use of immune checkpoint inhibitors for cancer: 68 cases from the Food and Drug Administration database and review. Eur J Haematol 2019;102:157–62.ArticlePubMed
- 10. Kong BY, Micklethwaite KP, Swaminathan S, Kefford RF, Carlino MS. Autoimmune hemolytic anemia induced by anti-PD-1 therapy in metastatic melanoma. Melanoma Res 2016;26:202–4.ArticlePubMed
- 11. Packman CH. The clinical pictures of autoimmune hemolytic anemia. Transfus Med Hemother 2015;42:317–24.ArticlePubMedPMC
- 12. Atiq O, Atiq SO, Atiq ZO, Patel V, Atiq MO, Atiq OT. Pembrolizumab-induced cold agglutinin disease. Am J Case Rep 2020;21:e924283.ArticlePubMedPMC
- 13. Choi J, Lee SY. Clinical characteristics and treatment of immune-related adverse events of immune checkpoint inhibitors. Immune Netw 2020;20:e9.ArticlePubMedPMC
- 14. Haanen JB, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28(Suppl 4):IV119–42.ArticlePubMed
- 15. Mahoney KM, Freeman GJ, McDermott DF. The next immune-checkpoint inhibitors: PD-1/PD-L1 blockade in melanoma. Clin Ther 2015;37:764–82.ArticlePubMedPMC
- 16. Nair R, Gheith S, Nair SG. Immunotherapy-associated hemolytic anemia with pure red-cell aplasia. N Engl J Med 2016;374:1096–7.ArticlePubMed
- 17. Palla AR, Kennedy D, Mosharraf H, Doll D. Autoimmune hemolytic anemia as a complication of nivolumab therapy. Case Rep Oncol 2016;9:691–7.ArticlePubMedPMC
References
Figure & Data
References
Citations

- Case Report: Life-threatening pancytopenia with tislelizumab followed by cerebral infarction in a patient with lung adenocarcinoma
Hang-Yu Gu, Jing-Wen Zhao, Yin-Shuang Wang, Zhuo-Nan Meng, Xiu-Ming Zhu, Fu-Wei Wang, Ai-Hong Zheng, Guo-Qing Wu
Frontiers in Immunology.2023;[Epub] CrossRef - Immunotherapy-associated Autoimmune Hemolytic Anemia
Steven R. Hwang, Antoine N. Saliba, Alexandra P. Wolanskyj-Spinner
Hematology/Oncology Clinics of North America.2022; 36(2): 365. CrossRef - Therapeutic plasma exchange in the management of immune checkpoint inhibitor‐associated immune‐related adverse effects: A review
Oluwatoyosi A. Onwuemene, Chizoba I. Nnoruka, Christopher J. Patriquin, Laura S. Connelly‐Smith
Transfusion.2022; 62(11): 2370. CrossRef - Diagnosis and management of cold agglutinin disease associated with low-grade B-cell lymphoma in a patient receiving pembrolizumab for lung cancer
Nabin Raj Karki, Peyton McElhone, Natasha Savage, Nagla Abdel Karim
BMJ Case Reports.2021; 14(8): e243751. CrossRef - Red Blood Cell Autoantibodies in Patients Treated with Immune Checkpoint Inhibitors
Eungjun Yoon, Tae Yeul Kim, Sun Kyoung Mun, Duck Cho
The Korean Journal of Blood Transfusion.2021; 32(3): 201. CrossRef

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite


