PubMed Central, CAS, DOAJ, KCI

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 41(1); 2024 > Article
-
Review article
Octacalcium phosphate, a promising bone substitute material: a narrative review -
Jooseong Kim1
 , Sukyoung Kim1
, Sukyoung Kim1 , Inhwan Song2
, Inhwan Song2
-
Journal of Yeungnam Medical Science 2024;41(1):4-12.
DOI: https://doi.org/10.12701/jyms.2023.00010
Published online: May 9, 2023
1HudensBio Co., Ltd., Gwangju, Korea
2Department of Anatomy, Yeungnam University College of Medicine, Daegu, Korea
- Corresponding author: Inhwan Song, MD, PhD Department of Anatomy, Yeungnam University College of Medicine, 170 Hyeonchung-ro, Nam-gu, Daegu 42415, Korea Tel: +82-53-640-6912 • Fax: +82-53-621-5083 • E-mail: ihsong@med.yu.ac.kr
Copyright © 2024 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Biomaterials have been used to supplement and restore function and structure by replacing or restoring parts of damaged tissues and organs. In ancient times, the medical use of biomaterials was limited owing to infection during surgery and poor surgical techniques. However, in modern times, the medical applications of biomaterials are diversifying owing to great developments in material science and medical technology. In this paper, we introduce biomaterials, focusing on calcium phosphate ceramics, including octacalcium phosphate, which has recently attracted attention as a bone graft material.
- 1. What are biomaterials?
- The term “biomaterials” is a complex concept with different interpretations that make it difficult to define in a few words. Different countries and agencies have various definitions [1-5]. From the simplest definition of “any materials used as implant” [1] to the more detail definition employed by the U.S. National Institute of Health that describes a biomaterial as “any substance or combination of substances, other than drugs, synthetic or natural in origin, which can be used for any period of time, which augments or replaces partially or totally any tissue, organ or function of the body, in order to maintain or improve the quality of life of the individual” [2]. Those definitions can be narrowly summarized as materials that can complement and restore functions by replacing or repairing parts of damaged tissues and organs in the living body, and in a broad sense, materials for diagnosing and treating diseases of the human body (Table 1).
- Biomaterials have been used for medical purposes since ancient times, as exemplified by, for example, sea shells as a substitute for missing teeth, skull prostheses using gold plates, and sutures using linen or catgut [5,6]; however, they were considered experimental because of poor surgical techniques and conditions [5].
- At the turn of the 19th century, on the basis of the pioneering microbiology of Louis Pasteur (AD 1822–1895) [7] and aseptic surgical technique of Joseph Lister (AD 1827–1912) [8], the improvements in surgical environments promoted research and development in material science. The concept of biomaterial biocompatibility was also established as biological reactions based on the properties of the biomaterial were understood [9]. Modern material engineering has led to the development of biomaterials and has achieved unprecedented development along with the medical technology developed during World War II [10-12].
- In this article, we introduce biomaterials focusing on calcium phosphate ceramics, including octacalcium phosphate (OCP), which has recently attracted attention.
- 2. Types and characteristics of biomaterials
- The most common way to classify biomaterials is to divide them into metals, ceramics, polymers, and composites, according to the type of material (Table 2). Another classification method is based on their interaction with the biological environment, dividing them into bioinert, bioactive, biodegradable, and resorbable materials (Table 3). Depending on their origin, they can also be classified as natural or synthetic materials [5,13].
- Metals are solid materials composed of elements such as Fe, Ni, Ti, Cr, Co, and Mo. Owing to the high toughness and ductility of metals, the manufacturing process is relatively simple and can easily be applied to various shapes [14,15]. Owing to their excellent mechanical properties, such as strength, abrasion, elasticity, and durability, metals are used as replacements for hard tissues such as teeth, bones, and joints. However, because of its low biocompatibility and susceptibility to corrosion, the material can lose its original properties, allowing the corrosion to penetrate the surrounding tissues and induce an inflammatory reaction [16]. In addition, metals can cause side effects due to metal ion elution; therefore, they are used in the form of alloys rather than pure metals [17].
- Ceramics are nonmetallic inorganic solid materials composed of elements such as Ca, P, K, Na, and Si. Owing to their excellent biocompatibility, high compressive strength, and wear resistance, they are used in dental implants, crowns, bone substitutes, and bone cements. However, the inherent brittleness of ceramics and the complicated manufacturing process compared to other materials are obstacles to their medical application [18,19].
- Polymers are substances or materials consisting of very large molecules called macromolecules that are composed of many repeating subunits. Polymers composed of a variety of materials are used in various medical fields because they can be manufactured with specific physical and chemical properties and relatively complex shapes [20]. However, the low mechanical strength, easy deformation, and deterioration of polymers are disadvantages for medical applications. Polymeric materials are used in a wider variety of fields than metal or ceramic materials, which are mainly used as substitutes for hard tissues. Polymers are frequently use in sutures, blood vessels, artificial joints, artificial tissues, and organs [21]. In addition to synthetic polymers, naturally derived polymers with excellent biocompatibility have been used. Natural polymers include collagen, gelatin, elastin, fibrin silk, hyaluronic acid, and heparin [22].
- Composite materials involve the complex use of two or more materials. In the case of coating a scaffold surface, composite materials possess both the physical strength of the base material and the high biocompatibility of the coating material; however, the manufacturing process is complicated for composites [23,24].
- 3. Requirements of biomaterials
- Because biomaterials replace damaged body parts, they must have adequate mechanical strength, chemical stability, and fatigue strength to maintain the biological function and shape of the tissues [25,26]. Another essential requirement for biomaterials that are inserted into the human body is biocompatibility [27]. Because most medical devices made of biomaterials are inserted into or make contact with the human body, the safety of cells and tissues around the inserted medical device is of paramount importance. The biomaterial should not be toxic or damage surrounding cells or tissues [28]. In addition, biomaterials must exhibit a high in vivo stability. Implanted materials are often easily hydrolyzed or deteriorated by the environment in the human body [29]. Therefore, it is necessary to improve the safety and efficacy of biomaterials in a living body through surface modification of the material.
- For the production of medical devices, biomaterials must be easy to process and sterilize. In general, it is difficult to manufacture medical devices aseptically. Thus, post-manufacturing sterilization is required, and the mechanical, chemical, and biological properties of the final biomaterials must not be altered by this process [30].
Introduction
- Biomaterials can be classified into organic and inorganic materials. Organic materials contain elements such as C, O, N, and H as their main components and include wood, paper, and natural fibers. Inorganic materials are nonorganic materials such as metals, stone, and soil, and are further subdivided into metallic and nonmetallic materials. Metallic materials are composed solely of metals such as Fe, Mg, Al, Ag, and Cu or a mixture (alloy) thereof. Materials in which the metallic elements are ionically bonded to anions, including oxygen, or covalently bonded to each other are called nonmetallic inorganic materials or ceramic materials [31]. A ceramic is a product obtained by forming a metal oxide or nonmetal compound and then sintering it at a high temperature.
- In general, pottery, cement, and glass made through sintering after the formation of nonmetallic inorganic materials are called traditional ceramics. High-purity ceramic powders are called fine ceramics and are used to make bioceramics, electronic products, or communication products [32]. Bioceramics are materials used to treat, reinforce, replace, or restore the functions of human tissues or organs for short or long periods of time. Bioceramics can be divided into bioinert, bioactive, and biodegradable types, according to their biological reactions. Bioinert bioceramics do not cause inflammation or toxicity when implanted into a living body and are bonded through the formation of surrounding fibrous tissue rather than directly binding to the surrounding living tissue. Alumina (Al2O3), zirconia (ZrO2), and carbon are included in this category [33]. Bioactive ceramics react with tissues and form chemical bonds directly, but only on the surface of the biomaterial. Bioglass and hydroxyapatite (HA) belong in this category. Biodegradable ceramics are chemically unstable biomaterials that gradually resorb and eventually disappear in the human body over time, and this resorbed space is filled with new human tissue. β-Tricalcium phosphate (β-TCP), OCP, metacalcium phosphate, and plaster of Paris (gypsum) are included in this category [33,34].
Bioceramics
- Calcium phosphate materials are composed of Ca, P, O, and H, and there are several types according to the atomic ratio of calcium to phosphorus [33] (Table 4). Because of their chemical similarity to human hard tissues, calcium phosphate-based bioceramics are attracting attention as they directly combine with hard tissue or regenerate bone without inflammatory reactions or new fibrous tissue formation when applied in vivo [35]. The term “bioceramics” refers to ceramic products manufactured using ceramic or precursor materials.
- Calcium phosphate-based ceramics have different thermodynamic properties depending on the ratio of calcium to phosphorus and show solubility differences in the human body or in solutions similar to body fluids. When various calcium phosphate-based ceramics are inserted into a living body, they exhibit different biological reactions with surrounding tissues [36]. In particular, calcium and phosphate ions released into the tissue greatly influences bone regeneration [37]. Calcium phosphate-based ceramics are classified as bioactive and biodegradable, according to their Ca/P ratios. However, the dissolution properties of calcium phosphate-based minerals depend on the pH of the solution. Furthermore, calcium phosphate-based ceramics exhibit excellent hydrophilicity and promote cell attachment and proliferation [38]. By replacing monovalent cations such as Na+, K+, and Li+, and divalent cations such as Sr2+, Ba2+, Pb2+, Mn2+, Sn2+, Zn2+, and Al3+, or by creating defects, the physical properties of minerals can be changed. They can also be subjected to high-temperature treatment to impart other properties. During high-temperature treatment, some calcium phosphate-based ceramics may change into minerals with low Ca/P ratios owing to the volatilization of phosphate [39].
- 1. Dicalcium phosphate dihydrate
- The mineral name for dicalcium phosphate dihydrate (DCPD) is brushite, and it is a component of calculi in the body. It is stable in an acidic environment and has a fast growth rate; therefore, it can be easily obtained in aqueous solutions. In addition, clinical results have shown that DCPD is more soluble than HA; therefore, DCPD is absorbed quickly in vivo and promotes bone formation [40]. Calcium is widely used in the food industry in addition to bone cement.
- 2. Hydroxyapatite
- Synthetic HA is more crystalline and richer in calcium ions than natural bone is. Although CO32– ions are partially substituted in the crystal structure of HA in natural bone, the basic chemical formula or crystal structure is very similar to that of synthetic HA [33,34]. Therefore, when an HA medical device is implanted into the body, an amorphous Ca-P mineral is formed on the surface of the HA, and after several months, an apatite layer similar to bone is formed. After a longer period (more than 6 months), direct bonding between the bone collagen fibers and HA occurs. Owing to these bioactive properties, HA is most commonly used as an artificial bone substitute. HA is more stable when the c-axis length in the unit cell is short and the pH is high. In contrast, solubility increases as pH decreases. When the OH of HA is substituted with F, the c-axis length in the unit cell shortens significantly, resulting in chemical stability. However, the use of fluoroapatite in large quantities is restricted because it must be within the permissible range of fluorine compounds in the body [41].
- Comparing the mechanical properties of natural bone and sintered artificial HA, the tensile strength of natural cortical bone is approximately 150 MPa and that of sintered artificial HA is approximately 100 MPa. Artificial HA has low strength and is prone to fracture, while natural bone has higher toughness than artificial bone because the collagen and nanometer-sized HA crystals form a complex. Therefore, synthetic HA is suitable when large loads are not required, and metal implants are commonly coated with HA for load-bearing applications. HA powder is easily synthesized by dry, wet, or hydrothermal treatments. Xenogeneic HA bone obtained by high-temperature treatment of natural bone has crystallinity and strength similar to that of synthetic HA, but its Ca/P ratio and surface morphology are similar to those of natural bone [42].
- 3. Tricalcium phosphate
- α-Tricalcium phosphate (α-TCP) possesses good biocompatibility, but it is chemically unstable and its biodegradation rate is fast. Therefore, in the past, cell attachment, proliferation, and differentiation on the surface of α-TCP biomaterials were difficult, limiting their applications as bone graft materials. However, in recent years, many studies have been conducted on the use of α-TCP as calcium phosphate-based cement. Thus, bone cement can be obtained by mixing α-TCP with other calcium phosphate-based materials. α-TCP can be obtained by heating β-TCP to a temperature of 1,300°C or higher and rapidly cooling it. α-TCP has the same chemical formula as β-TCP and both have excellent biocompatibilities; however, α-TCP is metastable at room temperature and is resorbed more rapidly in the body. When α-TCP is used as cement, calcium-deficient HA is finally produced after the cement reaction and is rapidly hydrolyzed in the human body [33,43].
- β-TCP, similar to α-TCP, does not exist naturally in the body; therefore, it is artificially synthesized and used. β-TCP is mainly produced by treating HA at high temperatures. Because β-TCP is rapidly biodegraded in the human body, it is used as a bone graft material by mixing with nonbiodegradable HA. This bone graft material is called biphasic calcium phosphate, which is mixed in varying proportions to control the biodegradation rate. In general, products with a mixing ratio of HA and β-TCP of 6:4 or 7:3 are common, and more types of these products are being commercialized than those manufactured with HA or β-TCP alone [34,44].
- Unlike α-TCP, β-TCP is used as resorbable filler for bone cement and is used for the purpose of controlling the rate of biodegradation. Bone cement hardener and β-TCP powder are mixed to form a slightly viscous paste, similar to toothpaste, which is then used to fill bone defects. Over time, the material hardens through cement reactions (hydration reactions, acid-base reactions, etc.). These products can be used clinically to fill bone defects more effectively than products in powder form [19].
- 4. Octacalcium phosphate
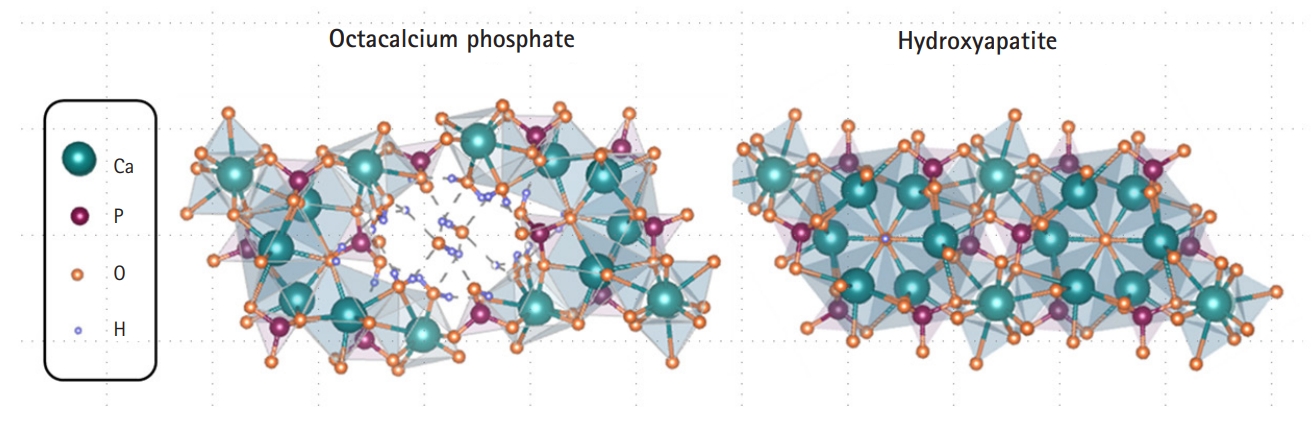
- OCP is a calcium phosphate-based material with a calcium to phosphorus ratio of 1.33 and is a precursor of biological HA in the human body. OCP is a thermodynamically unstable substance that is ultimately converted into HA, a stable substance, in the human environment [45,46]. The OCP crystal has a water layer between two layers of apatite, similar to the chemical formula (Ca8H2(PO4)6 5H2O) (Fig. 1). In a physiological environment, the water layer is removed from the OCP and the two apatite layers combine to form HA crystals (Fig. 1) [47,48]. Because of its crystallographic and chemical similarities, OCP has been proposed as a precursor to bioapatite crystals in bones and teeth [49]. The excellent osteoconductivity of OCP has been demonstrated in many animal studies [50,51]. Nevertheless, there have been limitations to many clinical applications due to the OCP bone graft production process and lack of mass production of OCP material [52].
- Owing to the problem of mass production of OCP materials, the Suzuki research group has been conducting investigations on combining OCP with other materials, such as collagen, gelatin, and alginate, rather than using pure OCP [53]. In a series of in vitro, animal, and clinical studies, this group demonstrated that bone graft materials containing OCP had much better bone regeneration than those made of HA or TCP [54,55]. For the first time, this research team recently developed a method for the mass production of OCP materials and published excellent animal and clinical research results for new bone regeneration with high-purity OCP bone graft materials.
- Anada et al. [56] examined the osteoblast differentiation capacity of OCP after seeding mouse bone marrow stromal ST-2 cells on dishes pre-coated with OCP and HA. When the ST-2 cells were cultured in OCP-coated wells, alkaline phosphatase (ALP) enzymatic activity gradually increased with increasing OCP concentrations. However, in the HA-coated group, ALP activity remained constant regardless of the HA content. In addition, OCP enhanced the expression of osteogenic markers, including osterix, collagen I, and ALP, on day 21 of culture. Therefore, OCP has the potential to improve osteoblast differentiation than HA.
- In another study, Shiwaku et al. [57] showed that large tartrate-resistant acid phosphatase–positive cells, representing multinucleated osteoclasts, were more frequently observed in cultures with biodegradable OCP or β-TCP discs than in those with non-degradable HA discs. The ability of OCP to form osteoclasts was almost the same as that of β-TCP, whereas the expression patterns of the coupling factors varied depending on the type of calcium phosphate. β-TCP and an HA/β-TCP mixture induced ephrin B2 and collagen triple helix repeat containing 1 expression, whereas OCP and HA/OCP mixtures promoted complement 3a expression.
- The superiority of OCP bone graft material was demonstrated in a comparative study between HA and β-TCP. Kamakura et al. [58] used a composite material by adding collagen to OCP, HA, and β-TCP and implanted it into a rat calvarial defect, followed by radiographic and histological examinations. They found that implanted OCP/collagen composites improved bone regeneration more than HA and β-TCP/collagen bone graft materials. New bone formation was also observed in the transplanted β-TCP/collagen group, and β-TCP resorption was not evident. In the case of HA/collagen, new bone formation was less pronounced than that of OCP/collagen and β-TCP/collagen. The authors concluded that the OCP/collagen composite material showed superior osteogenesis compared to the other materials.
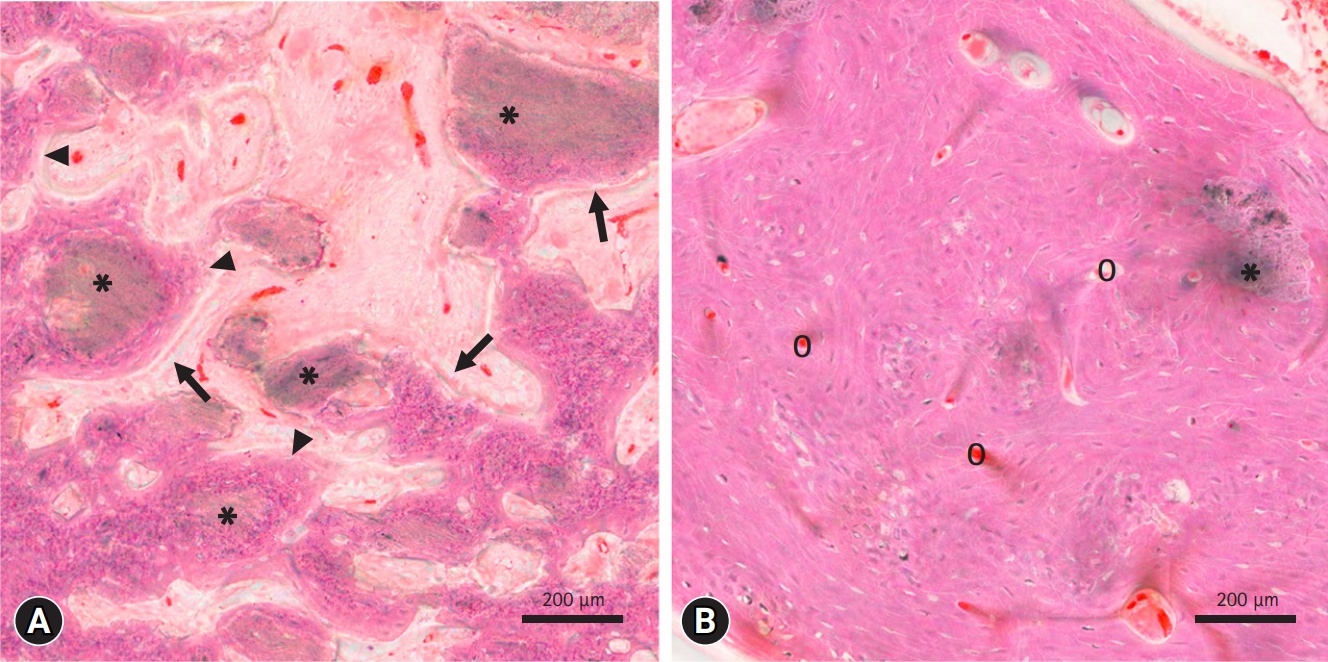
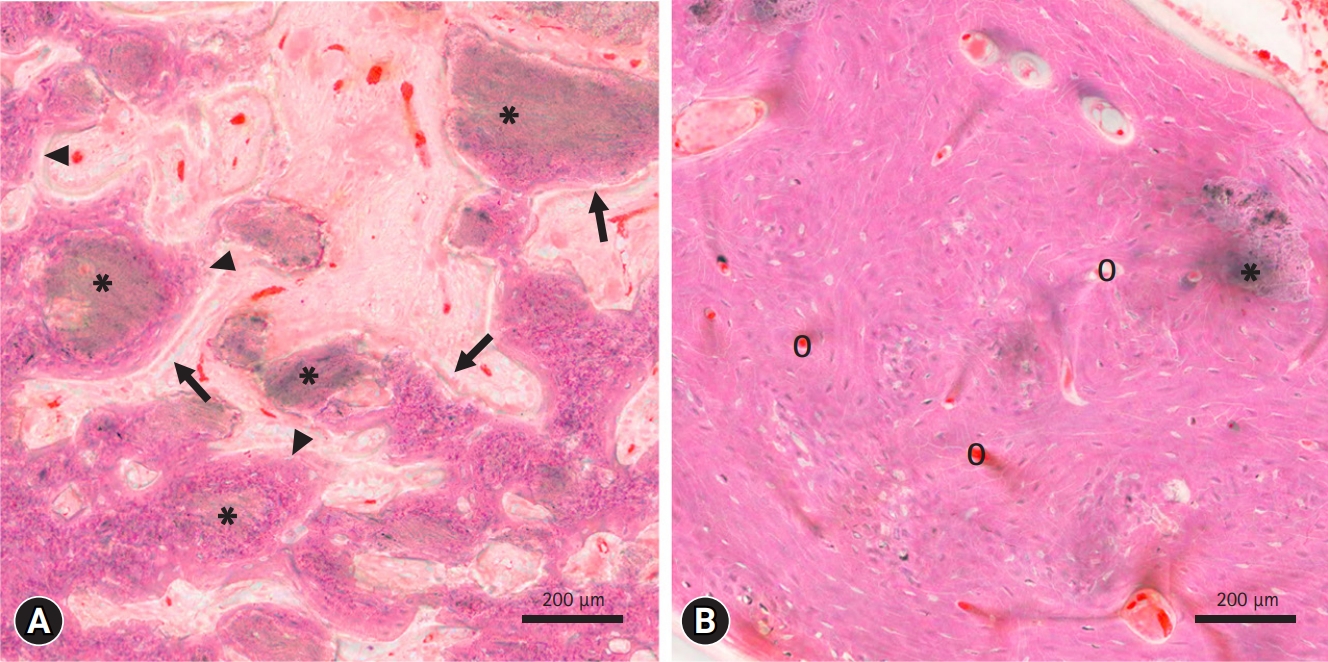
- In an animal study by Kim et al. [51], implanted pure OCP bone substitutes showed significant levels of osteogenic activity after 4 weeks. A high density of osteoblasts and new bone formation were observed around the implanted OCP granules (Fig. 2A). These histological findings strongly suggest that in addition to providing the basis for the crystallographic structure during bone regeneration, the OCP material itself promotes the homing and proliferation of bone-forming cells. After 12 weeks, most of the OCP bone graft material was resorbed, providing space for new bone formation (Fig. 2B).
- Several clinical trials have been conducted using OCP bone graft materials. OCP/collagen was placed in the nasal cavity and extraction socket of the left maxillary lateral incisor [59]. No infections or neoplastic lesions were observed at the treatment sites during the 7-year follow-up period. Moreover, no negative effects on neighboring teeth, such as mobility or loss, were confirmed. Hence, it can be stated that OCP/collagen was properly resorbed and replaced with new bone tissue. In addition, the newly formed bone due to the OCP/collagen showed affinity for and stably fixed the inserted dental implant.
- In another clinical study by Kim et al. [60], eight implants were placed in three patients who underwent maxillary sinus or alveolar bone grafting using OCP bone graft material. Except for mild swelling at the surgical site, none of the patients developed any postoperative complications. Four months after implantation, the implant stability quotient values were above 60 for all implants, indicating good implant stability. For site No. 16 of case 3, in which the maxillary sinus and ridge grafts were performed using OCP bone graft material, histological analysis revealed that new bone was deposited around the remaining grafted bone and the new bone was well integrated. No foreign body reactions or signs of inflammation were observed. Thus, the unique and excellent ability of OCP to generate new bone has been clinically and radiologically confirmed. Unlike HA and β-TCP, OCP appears to provide a starting site for new bone formation and eventually promotes bone regeneration [54].
Calcium phosphate bioceramics
- The results of studies on the excellent osteogenesis performance of OCP bone substitutes are of great significance in that they have emerged as a breakthrough that can overcome the disadvantages of existing naturally derived bones (allogeneic bone, xenogeneic bone) and synthetic bones. Despite the excellent capacity of OCP to generate new bone, its commercialization and applications have been limited in the past by the inability to produce high-purity OCP materials. Research on the mass production of OCP has been continuously conducted in countries around the world for the past 40 years, but only recently has the world's first high-purity OCP synthesis method and low-temperature manufacturing process for OCP bone been developed in Korea. Recently, domestic companies using these technologies have succeeded in commercializing bone substitutes based on OCP, and it is expected that the limitations of current bone graft materials can be overcome in clinical applications.
Conclusion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
This research was funded by a National Research Foundation of Korea Grant funded by the Korean Government (Ministry of Education; NRF-2017R1D1A3B03032090).
-
Author contributions
Conceptualization, Data curation: all authors; Formal analysis, Supervision: IS; Funding acquisition: SK, IS; Validation: SK; Writing-original draft: JK, SK; Writing-review & editing: SK, IS.
Article information
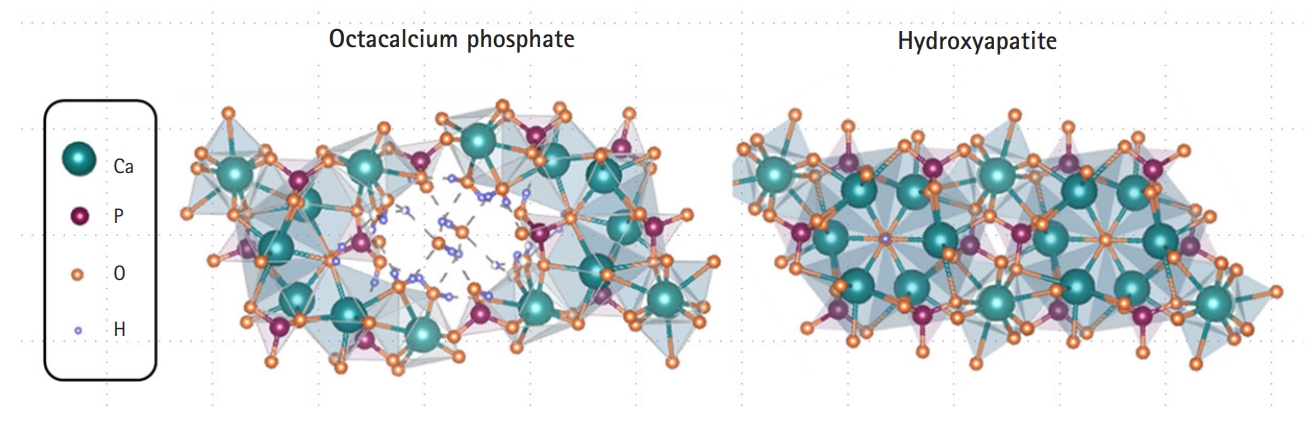
Adapted from Parida et al. [61] according to the Creative Commons License.
- 1. Cohen J. Biomaterials in orthopedic surgery. Am J Surg 1967;114:31–41.ArticlePubMed
- 2. National Institute of Health (NIH). Clinical applications of biomaterials: National Institute of Health Consensus Development Conference statement [Internet]. Bethesda, MD: NIH; 1982 [cited 2023 Jan 24]. https://consensus.nih.gov/1982/1982Biomaterials034html.htm.
- 3. Williams DF; European Society for Biomaterials. Definitions in biomaterials: proceedings of a consensus conference of the European Society for Biomaterials, Chester, England, March 3-5, 1986. Amsterdam: Elsevier; 1987.
- 4. Williams DF. The Williams dictionary of biomaterials. Liverpool: Liverpool University Press; 1999.
- 5. Marin E, Boschetto F, Pezzotti G. Biomaterials and biocompatibility: an historical overview. J Biomed Mater Res A 2020;108:1617–33.ArticlePubMedPDF
- 6. Arnott R. Surgical practice in the prehistoric Aegean. Medizinhist J 1997;32:249–78.PubMed
- 7. Bordenave G. Louis Pasteur (1822-1895). Microbes Infect 2003;5:553–60.ArticlePubMed
- 8. Newsom SW. Pioneers in infection control-Joseph Lister. J Hosp Infect 2003;55:246–53.ArticlePubMed
- 9. Williams DF. On the mechanisms of biocompatibility. Biomaterials 2008;29:2941–53.ArticlePubMed
- 10. Datta LP, Manchineella S, Govindaraju T. Biomolecules-derived biomaterials. Biomaterials 2020;230:119633.ArticlePubMed
- 11. Basu B, Gowtham NH, Xiao Y, Kalidindi SR, Leong KW. Biomaterialomics: data science-driven pathways to develop fourth-generation biomaterials. Acta Biomater 2022;143:1–25.ArticlePubMed
- 12. Enderle JD, Bronzino JD. Introduction to biomedical engineering. Amsterdam: Elsevier/Academic Press; 2012.
- 13. Kargozar S, Ramakrishna S, Mozafari M. Chemistry of biomaterials: future prospects. Curr Opin Biomed Eng 2019;10:181–90.Article
- 14. Yang K, Zhou C, Fan H, Fan Y, Jiang Q, Song P, et al. Bio-functional design, application and trends in metallic biomaterials. Int J Mol Sci 2017;19:24.ArticlePubMedPMC
- 15. Prasad K, Bazaka O, Chua M, Rochford M, Fedrick L, Spoor J, et al. Metallic biomaterials: current challenges and opportunities. Materials (Basel) 2017;10:884.ArticlePubMedPMC
- 16. Manivasagam G, Dhinasekaran D, Rajamanickam A. Biomedical implants: corrosion and its prevention-a review. Recent Patents Corros Sci 2010;2:40–54.Article
- 17. Szczęsny G, Kopec M, Politis DJ, Kowalewski ZL, Łazarski A, Szolc T. A review on biomaterials for orthopaedic surgery and traumatology: from past to present. Materials (Basel) 2022;15:3622.ArticlePubMedPMC
- 18. Vallet-Regí M. Ceramics for medical applications. J Chem Soc Dalton Trans 2001;(2):97–108.
- 19. Kenny SM, Buggy M. Bone cements and fillers: a review. J Mater Sci Mater Med 2003;14:923–38.PubMed
- 20. Goor OJ, Hendrikse SI, Dankers PY, Meijer EW. From supramolecular polymers to multi-component biomaterials. Chem Soc Rev 2017;46:6621–37.ArticlePubMed
- 21. Gribova V, Crouzier T, Picart C. A material’s point of view on recent developments of polymeric biomaterials: control of mechanical and biochemical properties. J Mater Chem 2011;21:14354–66.ArticlePubMedPMC
- 22. Ko HF, Sfeir C, Kumta PN. Novel synthesis strategies for natural polymer and composite biomaterials as potential scaffolds for tissue engineering. Philos Trans A Math Phys Eng Sci 2010;368:1981–97.ArticlePubMedPMCPDF
- 23. Leu Alexa R, Cucuruz A, Ghițulică CD, Voicu G, Stamat Balahura LR, Dinescu S, et al. 3D printable composite biomaterials based on GelMA and hydroxyapatite powders doped with cerium ions for bone tissue regeneration. Int J Mol Sci 2022;23:1841.ArticlePubMedPMC
- 24. Wei Q, Becherer T, Angioletti-Uberti S, Dzubiella J, Wischke C, Neffe AT, et al. Protein interactions with polymer coatings and biomaterials. Angew Chem Int Ed Engl 2014;53:8004–31.ArticlePubMed
- 25. Yin W, Chen M, Bai J, Xu Y, Wang M, Geng D, et al. Recent advances in orthopedic polyetheretherketone biomaterials: material fabrication and biofunction establishment. Smart Mater Med 2022;3:20–36.Article
- 26. Rehman M, Madni A, Webster TJ. The era of biofunctional biomaterials in orthopedics: what does the future hold? Expert Rev Med Devices 2018;15:193–204.ArticlePubMed
- 27. Bryers JD, Giachelli CM, Ratner BD. Engineering biomaterials to integrate and heal: the biocompatibility paradigm shifts. Biotechnol Bioeng 2012;109:1898–911.ArticlePubMedPMC
- 28. Helmus MN, Gibbons DF, Cebon D. Biocompatibility: meeting a key functional requirement of next-generation medical devices. Toxicol Pathol 2008;36:70–80.ArticlePubMedPDF
- 29. Balamurugan A, Rajeswari S, Balossier G, Rebelo AH, Ferreira JM. Corrosion aspects of metallic implants: an overview. Mater Corros 2008;59:855–69.Article
- 30. Harrington RE, Guda T, Lambert B, Martin J. Sterilization and disinfection of biomaterials for medical devices. In: Wagner WR, Sakiyama-Elbert SE, Zhang G, Yaszemski MJ, editors. Biomaterials science. 4th ed. Cambridge: Academic Press; 2020. p. 1431–46.
- 31. Sukumaran VG, Bharadwaj N. Ceramics in dental applications. Trends Biomater Artif Organs 2006;20:7–12.
- 32. DeGarmo EP, Black JT, Kohser RA. DeGarmo's materials and processes in manufacturing. 12th ed. Hoboken, NJ: John Wiley & Sons; 2017.
- 33. Kumar P, Dehiya BS, Sindhu A. Bioceramics for hard tissue engineering applications: a review. Int J Appl Eng Res 2018;13:2744–52.
- 34. Best SM, Porter AE, Thian ES, Huang J. Bioceramics: past, present and for the future. J Eur Ceram Soc 2008;28:1319–27.Article
- 35. Dorozhkin SV. Calcium orthophosphates as bioceramics: state of the art. J Funct Biomater 2010;1:22–107.ArticlePubMedPMC
- 36. Vallet-Regi M, González-Calbet JM. Calcium phosphates as substitution of bone tissues. Prog Solid State Chem 2004;32:1–31.Article
- 37. Samavedi S, Whittington AR, Goldstein AS. Calcium phosphate ceramics in bone tissue engineering: a review of properties and their influence on cell behavior. Acta Biomater 2013;9:8037–45.ArticlePubMed
- 38. Hajiali F, Tajbakhsh S, Shojaei A. Fabrication and properties of polycaprolactone composites containing calcium phosphate-based ceramics and bioactive glasses in bone tissue engineering: a review. Polym Rev 2018;58:164–207.Article
- 39. Gaharwar AK, Cross LM, Peak CW, Gold K, Carrow JK, Brokesh A, et al. 2D Nanoclay for biomedical applications: regenerative medicine, therapeutic delivery, and additive manufacturing. Adv Mater 2019;31:e1900332.ArticlePubMedPMCPDF
- 40. Alge DL, Santa Cruz G, Goebel WS, Chu TM. Characterization of dicalcium phosphate dihydrate cements prepared using a novel hydroxyapatite-based formulation. Biomed Mater 2009;4:025016.ArticlePubMed
- 41. Cacciotti I. Cationic and anionic substitutions in hydroxyapatite. In: Antoniac I, editors. Handbook of bioceramics and biocomposites. Berlin: Springer; 2016. p. 145–211.
- 42. Fu Q, Saiz E, Rahaman MN, Tomsia AP. Toward strong and tough glass and ceramic scaffolds for bone repair. Adv Funct Mater 2013;23:5461–76.ArticlePubMedPMC
- 43. Carrodeguas RG, De Aza S. α-Tricalcium phosphate: synthesis, properties and biomedical applications. Acta Biomater 2011;7:3536–46.ArticlePubMed
- 44. Bohner M, Santoni BLG, Döbelin N. β-tricalcium phosphate for bone substitution: synthesis and properties. Acta Biomater 2020;113:23–41.ArticlePubMed
- 45. Wang L, Nancollas GH. Calcium orthophosphates: crystallization and dissolution. Chem Rev 2008;108:4628–69.ArticlePubMedPMC
- 46. Combes C, Rey C. Amorphous calcium phosphates: synthesis, properties and uses in biomaterials. Acta Biomater 2010;6:3362–78.ArticlePubMed
- 47. Brown WE, Schroeder LW, Ferris JS. Interlayering of crystalline octacalcium phosphate and hydroxylapatite. J Phys Chem 1979;83:1385–8.Article
- 48. Black JD, Tadros BJ. Bone structure: from cortical to calcium. Orthop Trauma 2020;34:113–9.Article
- 49. Mathew M, Takagi S. Structures of biological minerals in dental research. J Res Natl Inst Stand Technol 2001;106:1035–44.ArticlePubMedPMC
- 50. Suzuki O. Octacalcium phosphate: osteoconductivity and crystal chemistry. Acta Biomater 2010;6:3379–87.ArticlePubMed
- 51. Kim J, Kim S, Song I. Biomimetic octacalcium phosphate bone has superior bone regeneration ability compared to xenogeneic or synthetic bone. Materials (Basel) 2021;14:5300.ArticlePubMedPMC
- 52. Suzuki O, Shiwaku Y, Hamai R. Octacalcium phosphate bone substitute materials: comparison between properties of biomaterials and other calcium phosphate materials. Dent Mater J 2020;39:187–99.ArticlePubMed
- 53. Kawai T, Anada T, Honda Y, Kamakura S, Matsui K, Matsui A, et al. Synthetic octacalcium phosphate augments bone regeneration correlated with its content in collagen scaffold. Tissue Eng Part A 2009;15:23–32.ArticlePubMed
- 54. Sakai S, Anada T, Tsuchiya K, Yamazaki H, Margolis HC, Suzuki O. Comparative study on the resorbability and dissolution behavior of octacalcium phosphate, β-tricalcium phosphate, and hydroxyapatite under physiological conditions. Dent Mater J 2016;35:216–24.ArticlePubMed
- 55. Kamakura S, Sasano Y, Shimizu T, Hatori K, Suzuki O, Kagayama M, et al. Implanted octacalcium phosphate is more resorbable than beta-tricalcium phosphate and hydroxyapatite. J Biomed Mater Res 2002;59:29–34.ArticlePubMed
- 56. Anada T, Kumagai T, Honda Y, Masuda T, Kamijo R, Kamakura S, et al. Dose-dependent osteogenic effect of octacalcium phosphate on mouse bone marrow stromal cells. Tissue Eng Part A 2008;14:965–78.ArticlePubMed
- 57. Shiwaku Y, Tsuchiya K, Xiao L, Suzuki O. Effect of calcium phosphate phases affecting the crosstalk between osteoblasts and osteoclasts in vitro. J Biomed Mater Res A 2019;107:1001–13.ArticlePubMedPDF
- 58. Kamakura S, Sasaki K, Honda Y, Masuda T, Anada T, Kawai T, et al. Differences of bone regeneration by various calcium phosphate/collagen composites. Key Eng Mater 2008;361-3:1229–32.Article
- 59. Kawai T, Echigo S, Matsui K, Tanuma Y, Takahashi T, Suzuki O, et al. First clinical application of octacalcium phosphate collagen composite in human bone defect. Tissue Eng Part A 2014;20:1336–41.ArticlePubMedPMC
- 60. Kim JS, Jang TS, Kim SY, Lee WP. Octacalcium phosphate bone substitute (Bontree®): from basic research to clinical case study. Appl Sci 2021;11:7921.Article
- 61. Parida P, Behera A, Mishra SC. Classification of biomaterials used in medicine. Int J Adv Appl Sci 2012;1:31–5.Article
References
Figure & Data
References
Citations

- Development of Hydroxyapatite Coatings for Orthopaedic Implants from Colloidal Solutions: Part 1—Effect of Solution Concentration and Deposition Kinetics
Bríd Murphy, Mick A. Morris, Jhonattan Baez
Nanomaterials.2023; 13(18): 2577. CrossRef

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite