Indexed in: ESCI, Scopus, PubMed,
PubMed Central, CAS, DOAJ, KCI
PubMed Central, CAS, DOAJ, KCI
FREE article processing charge

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 41(2); 2024 > Article
-
Original article
Impact of COVID-19 on the development of major mental disorders in patients visiting a university hospital: a retrospective observational study -
Hee-Cheol Kim

-
Journal of Yeungnam Medical Science 2024;41(2):86-95.
DOI: https://doi.org/10.12701/jyms.2023.01256
Published online: February 6, 2024
Department of Psychiatry and Brain Research Institute, Keimyung University School of Medicine, Daegu, Korea
- Corresponding author: Hee-Cheol Kim, MD, PhD Department of Psychiatry, Keimyung University School of Medicine, 1095 Dalgubeol-daero, Dalseo-gu, Daegu 42601, Korea Tel: +82-53-258-7916 • Fax: +82-53-258-4882 • E-mail: mdhck@dsmc.or.kr
• Received: November 16, 2023 • Revised: December 17, 2023 • Accepted: December 26, 2023
© 2024 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,493 Views
- 48 Download
Abstract
-
Background
- This study aimed to investigate the impact of coronavirus disease 2019 (COVID-19) on the development of major mental disorders in patients visiting a university hospital.
-
Methods
- The study participants were patients with COVID-19 (n=5,006) and those without COVID-19 (n=367,162) registered in the database of Keimyung University Dongsan Hospital and standardized with the Observational Medical Outcomes Partnership Common Data Model. Data on major mental disorders that developed in both groups over the 5-year follow-up period were extracted using the FeederNet computer program. A multivariate Cox proportional hazards model was used to estimate the hazard ratio (HR) and 95% confidence interval (CI) for the incidence of major mental disorders.
-
Results
- The incidences of dementia and sleep, anxiety, and depressive disorders were significantly higher in the COVID-19 group than in the control group. The incidence rates per 1,000 patient-years in the COVID-19 group vs. the control group were 12.71 vs. 3.76 for dementia, 17.42 vs. 7.91 for sleep disorders, 6.15 vs. 3.41 for anxiety disorders, and 8.30 vs. 5.78 for depressive disorders. There was no significant difference in the incidence of schizophrenia or bipolar disorder between the two groups. COVID-19 infection increased the risk of mental disorders in the following order: dementia (HR, 3.49; 95% CI, 2.45–4.98), sleep disorders (HR, 2.27; 95% CI, 1.76–2.91), anxiety disorders (HR, 1.90; 95% CI, 1.25–2.84), and depressive disorders (HR, 1.54; 95% CI, 1.09–2.15).
-
Conclusion
- This study showed that the major mental disorders associated with COVID-19 were dementia and sleep, anxiety, and depressive disorders.
- Coronavirus disease 2019 (COVID-19), caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has significantly affected mental health worldwide. The pandemic has created a unique set of stressors and challenges that have contributed to the exacerbation of existing mental health conditions and the onset of new ones [1,2]. COVID-19 is associated with several mental health issues. The uncertainty surrounding the virus, fear of infection, and concerns about the health of loved ones have led to increased anxiety and stress among individuals. Lockdowns, social isolation, and economic hardships have also contributed to increased stress levels. The pandemic has been associated with an increase in depressive symptoms. Factors such as social isolation, employment loss, and death of loved ones play a role in the development or exacerbation of depression.
- Lockdowns, quarantine measures, and social distancing have led to increased social isolation and loneliness, which can contribute to mental health issues, especially among vulnerable populations, such as the elderly. The pandemic has disrupted mental health services, making it challenging for some individuals to access the care and support they need [3]. Healthcare workers and individuals who contracted the virus may be at risk of developing post-traumatic stress disorder because of the traumatic experiences they endured during the pandemic [4]. Financial stress resulting from job loss or economic instability can contribute to mental health problems including anxiety and depression [5]. Disruption of daily routines and the closure of gyms, recreational facilities, and other outlets for physical and mental well-being have impacted mental health. Stigmatization of individuals who have contracted the virus or are frontline healthcare workers has been reported and can lead to mental health challenges for those affected [6].
- It is important to note that the impact of the pandemic on mental health varies widely from person to person. While some individuals have developed resilience and coping mechanisms, others face significant challenges [7]. Patients visiting hospitals may have weaker resilience and coping mechanisms than individuals in the general population [8]. COVID-19 primarily affects the respiratory system but can have a wide range of effects on various organs and systems in the body, including the brain. Emerging evidence suggests that COVID-19 is associated with an increased risk of cognitive and neurological symptoms, raising concerns about its potential impact on dementia [9-11]. Most patients recover from COVID-19 within 2 weeks without long-term symptoms. However, many people continue to experience memory problems or cloudy thinking, even without other symptoms of long COVID. These neurological symptoms can last for weeks or months after an infection. A recent review has highlighted the high frequency of cognitive impairment following the COVID-19 outbreak [11].
- Previous studies have shown that COVID-19 influences the development and worsening of major mental health disorders. This study investigated the impact of COVID-19 on the development of major mental disorders in patients visiting university hospitals.
Introduction
- Ethical statements
- This study was approved by the Institutional Review Board (IRB) of Keimyung University Dongsan Hospital (IRB No: DSMC 2023-10-064). The requirement of informed consent was waived.
- 1. Data sources
- This was a single-center, retrospective cohort study of Korean patients (n=513,262) who visited Keimyung University Dongsan Hospital between November 1, 2018 and October 31, 2023 (study period). The Observational Medical Outcomes Partnership Common Data Model (OMOP CDM) version 5.3.1 database and open-source Observational Health Data Sciences and Informatics (OHDSI) software were used in the investigation [12]. The OHDSI organization provides a large selection of open-source tools that handle different analytic scenarios using patient-level observational data. An open community data standard called the OMOP CDM was created to standardize the composition and organization of observational data and facilitate effective analyses that can yield solid support.
- 2. Study design
- To investigate the association between COVID-19 and major mental disorders, this study was conducted on patients with COVID-19 (target group: n=5,006; mean age, 42.02±22.47 years) and those without COVID-19 (comparator: n=367,162; mean age, 50.01±22.75 years) who were registered in the database of Keimyung University Dongsan Hospital standardized with the OMOP CDM. This study was conducted using a medical real-world data (RWD) platform called FeederNet, which is the Federated E-Health Big Data for Evidence Renovation Network. FeederNet standardizes and de-identifies electronic medical record data from approximately 50 large general hospitals in Korea using the CDM, and each piece of data is safely stored without the risk of personal information being leaked. FeederNet is an RWD platform that allows distributed multi-institutional research to be managed in hospitals. Researchers who have registered as members of FeederNet can log into the FeederNet server (https://feedernet.com/member/main) to conduct research. The FeederNet computer application was used to extract the current study data [13]. The OMOP CDM schema was used to map all patients by providing a consistent format for healthcare data and standardizing the clinical coding systems. This allowed the analysis code to be shared among the network participants’ datasets.
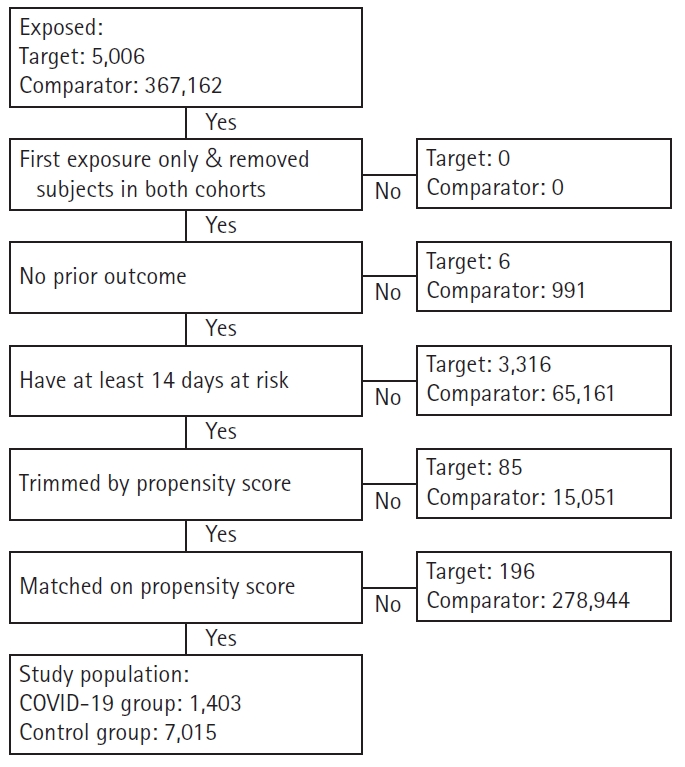
- During the study period, the index date was defined as the first day of COVID-19 diagnosis in the COVID-19 group and the first day of hospital visit in the control group. Both groups were censored at the end of the database, at the time of mental disorder identification. Patients with a history of any mental disorder prior to study enrollment, cases in which any mental disorder occurred before the index date, and cases in which the duration of the mental disorder during the follow-up period was less than 14 days were excluded from the study. Through 1:5 matching using large-scale propensity score matching (PSM) modeling [14], a control group with demographic and clinical characteristics similar to those of the target group was extracted from the comparator. The covariates used in the PSM included age, sex, prior conditions, and Romano et al. [15]’s adaptation of the Charlson Comorbidity Index. After PSM, a standardized difference of <0.25 for every covariate was considered negligible [16]. The target group, which was matched to the control group, was classified as the COVID-19 group.
- The numbers of patients in the COVID-19 and control groups, respectively, extracted for investigating the incidence rates for nine major mental disorders were as follows: 1,403 and 7,015 for depressive disorders (Fig. 1); 1,405 and 7,025 for anxiety disorders; 1,408 and 7,040 for bipolar disorders; 1,407 and 7,035 for schizophrenia; 1,403 and 7,015 for sleep disorders; 1,409 and 7,045 for eating disorders; 1,407 and 7,035 for psychoactive substance dependence; and 1,220 and 6,098 for dementia (additionally excluding patients with a history of dementia prior to the index date). Only the flowchart of the data extraction process for depressive disorders is provided because the extraction processes for all nine mental disorders were nearly identical.
- 3. Outcomes
- The outcome event in this study was the initial diagnosis of a major mental disorder. The outcome variable used in data analysis was the number of new cases of major mental disorders. The major mental disorders investigated in this study included anxiety, depressive, and bipolar disorders; schizophrenia; sleep disorders; eating disorders; psychoactive substance dependence; and dementia. The Systematized Nomenclature of Medicine Clinical Terms (SNOMED CT) was used to create diagnostic codes [17], and these definitions were mapped to other terminologies, such as the Korean Classification of Disease 8th version, which is an updated version of the International Classification of Diseases 10th revision [18]. SNOMED CT is a comprehensive clinical terminology and coding system used in healthcare and medical informatics. It is intended to facilitate the standardization and consistency of clinical and medical information sharing between information systems and healthcare practitioners. The SNOMED CT codes for major mental disorders included in this study are as follows: anxiety disorders, 197480006; depressive disorders, 35489007; bipolar disorders, 13746004; schizophrenia, 58214004; sleep disorders, 39898005; eating disorders, 72366004; psychoactive substance dependence, 2403008; and dementia, 52448006. Each SNOMED CT code includes all low-level concepts (descendants). The primary outcome was the incidence of major mental disorders that occurred at least 30 days after the index date. The criterion for the onset of various mental disorders was defined as a disease that persisted for at least 14 days.
- 4. Statistical analysis
- The open-source OHDSI CohortMethod R package was used in this study, and the Cyclops R package was used for large-scale analytics. FeederNet was utilized for analysis, and ATLAS version 2.7.5 was the computer software employed. Researchers performed scientific analyses on standardized observational data transformed into OMOP CDM using the open-source ATLAS software package. Differences in the distribution of demographic and clinical characteristics between the two groups before and after PSM are presented as percentages and standardized differences. Categorical variables were compared between the two groups before and after PSM using the chi-square test. The log-rank test was used to evaluate the survival curves between the two groups, and the Kaplan-Meier survival analysis was applied to ascertain the difference in probability of being mental disorder-free between the two groups. Adjusted hazard ratios (aHRs) were calculated using a multivariate Cox proportional hazards model. The difference in survival time between the two groups was calculated using a multivariate Cox proportional hazards model that produced aHRs and 95% confidence intervals (CIs). After adjusting for baseline covariates, a propensity model was fitted, and the propensity scores obtained were used to match the COVID-19 and control groups using variable ratio matching [19]. A p-value of less than 0.05 was regarded as a statistically significant difference.
Methods
- 1. Cohort characteristics
- The clinical characteristics of the COVID-19 and control groups were almost identical in each analysis that evaluated the incidence of major mental disorders. Therefore, only the characteristics in the analysis of the incidence of depressive disorders are presented as representative data. The baseline characteristics of the study population before and after PSM are presented in Tables 1 to 5. After PSM, the absolute standardized differences in all baseline characteristics between the COVID-19 and control groups were <0.25.
- 2. Outcome assessment and survival curves
- The maximum “time at risk” days in this study were 1,796 days in both the COVID-19 and control groups. The number of subjects, follow-up time, number of outcome events, event incidence rate, and minimum detectable relative risk are presented in Table 6. The observed HR for the risk of major mental disorders in the COVID-19 group relative to that in the control group was 1.54 (95% CI, 1.09–2.15; p<0.05) for depressive disorders, 1.90 (95% CI, 1.25–2.84; p<0.01) for anxiety disorders, 1.11 (95% CI, 0.49–2.25; p=0.80) for bipolar disorders, 2.27 (95% CI, 1.76–2.91; p<0.01) for sleep disorders, and 3.49 (95% CI, 2.45–4.98; p<0.01) for dementia (Fig. 2). The HR for schizophrenia, eating disorders, and psychoactive substance dependence could not be calculated because the incidence rate was too low. The proportional hazard assumption underlying the Cox regression model was examined graphically and cases in which this assumption was not met in the survival analysis were excluded. The Kaplan-Meier curves for the risk of mental disorders between the COVID-19 and control groups over the 5-year follow-up period are shown in Fig. 3.
Results
- This study showed that the major mental disorders associated with COVID-19 were dementia and sleep, anxiety, and depressive disorders. In this study, the cumulative incidence (probability) of a first diagnosis of dementia in the COVID-19 group was 5.25%. Relative to the control group, the HR for dementia in the COVID-19 group was 3.49. Taquet et al. [20] reported that the probability of receiving a dementia diagnosis for the first time within 14 to 90 days following a COVID-19 diagnosis was 1.6% (95% CI, 1.2–2.1) in people older than 65 years. In a retrospective cohort study, Gollop et al. [21] examined patients aged 65 years or older who initially received a diagnosis of acute upper respiratory infection (URI) or COVID-19. The study found that after a 12-month follow-up, 1.84% of patients with COVID-19 and 1.78% of patients with URI had dementia. However, the present study found a higher cumulative incidence of dementia after COVID-19 than the two previous studies. Regarding this difference in the cumulative incidence of dementia, the dependence on patient characteristics cannot be ruled out. Because this study investigated the incidence of dementia in patients who visited a hospital, it may differ from other studies that have investigated the general population. In contrast, Freudenberg-Hua et al. [22] reported that the 1-year incidence of post-COVID-19 dementia was 12.7%, which was a significantly higher cumulative incidence than that reported in this study (5.25%). However, different selection criteria were used for the study subjects in both studies. In contrast to Freudenberg-Hua et al.’s study [22], which only investigated patients aged 65 years or older who were hospitalized, our study evaluated patients of all ages who visited a hospital, regardless of hospitalization status. When our study was reanalyzed for patients aged 65 years or older, the cumulative incidence of dementia was found to be 10.36% (60 of 579), a significant increase. These results showed that the risk of dementia after SARS-CoV-2 infection was higher in patients who visited hospitals, especially in those hospitalized with COVID-19, than in the general population.
- In this study, dementia had the highest HR for major mental disorders. Taquet et al. [20] also reported that the incidence of dementia is higher than that of other mental disorders (e.g., depression, anxiety disorder, and insomnia). These results may be related to differences in the clinical course of each disease. A previous study found that while the risk of mood and anxiety disorders decreases 1 to 2 months after COVID-19, the risk of dementia and cognitive impairment may persist for up to 2 years following infection [23]. Taquet et al. [23] explained that the persistent increase in the risk of cognitive deficits and dementia after COVID-19 suggests that the underlying mechanism must continue to operate even after the acute infection has passed. They also explained that mood and anxiety disorders notably followed a different pattern in which their heightened risk decreased in less than 2 months, and their cumulative incidence did not increase after 2 years. In this study by Taquet et al. [23], the HRs for mental disorders 6 months after COVID-19 were estimated to be 1.13 for anxiety disorders, 1.08 for mood disorders, 1.13 for insomnia, and 1.33 for dementia. Xie et al. [24] also analyzed the risk of mental health outcomes in patients with COVID-19 versus a control group and found that the 1-year HRs for anxiety disorders, depressive disorders, sleep disorders, and neurocognitive decline were 1.35, 1.39, 1.41, and 1.80, respectively. Similar to previous studies, the present study showed that the HR for dementia (3.49) was the highest among the mental disorders.
- To determine whether this result is related to the clinical course of the disease described by Taquet et al. [23], we further investigated the incidence rate per 1,000 person-years of mental disorders in the COVID-19 and control groups divided into two periods, within 1 year and 1 year after the index date. We found that the incidence rate of anxiety, depression, and sleep disorders, as well as dementia in the COVID-19 group declined 1 year after the index date. The incidence rate declines in the COVID-19 group were as follows: depressive disorder (66.4%), anxiety disorder (51.4%), sleep disorder (32.1%), and dementia (26.5%). However, the rates of decline were similar to those observed in the control group. Therefore, it is inappropriate to interpret the high HR for dementia in this study as solely based on the clinical course of the disease. However, this study showed that the HR for dementia (4.66) was much higher than those for other mental disorders (1.51–1.63) within 1 year after the index date. These results suggest that the impact of COVID-19 on dementia is much higher than the impact of COVID-19 on other mental disorders.
- COVID-19 is associated with an increased risk of cognitive impairment and neurological complications [25,26]. The risk of dementia is increased by COVID-19 according to several recent studies [9-11]. The chronic inflammation and vascular damage caused by COVID-19 increase the risk of cognitive decline and dementia [27]. This study confirmed that COVID-19 raises the risk of dementia by showing that the COVID-19 group’s risk of dementia was 3.49 times higher than the control group’s risk when followed up for up to 5 years following COVID-19 infection. Dementia investigated in this study comprised all types, including vascular dementia and that caused by neurodegenerative diseases such as Alzheimer disease and Parkinson disease.
- Other mental disorders related to COVID-19 identified in this study included sleep, anxiety, and depressive disorders. However, the incidences of bipolar disorder and schizophrenia in both the COVID-19 and control groups were so low that it was difficult to assess their association with COVID-19. In addition, there were no cases of eating disorders or psychoactive substance dependence during the follow-up period in either the COVID-19 or control group, making it impossible to evaluate the relationship between these diseases and COVID-19.
- Numerous sleep-related problems have been linked to COVID-19, both in the acute stage of the illness and as part of its chronic symptoms. Some individuals with COVID-19 may have trouble falling or staying asleep, often due to the discomfort, pain, or anxiety associated with the illness. COVID-19 can cause respiratory problems that may worsen sleep apnea or lead to temporary episodes of breathing difficulties during sleep. Some individuals with long COVID histories report persistent sleep disturbances as a part of their ongoing symptoms. These conditions include insomnia, excessive daytime sleepiness, and irregular sleep patterns [28,29]. In this study, the incidence of sleep disorders was higher in the COVID-19 group than in the control group; however, a detailed analysis by sleep disorder type was not possible because of the small number of participants.
- Infectious diseases and anxiety disorders are often linked and place significant burdens on individuals, families, and society [30]. The influence of COVID-19 on mental health, specifically on anxiety disorders, has been substantial. The pandemic and its associated challenges have led to increased levels of anxiety and the exacerbation of preexisting anxiety disorders [31]. Depression is the most common mental illness worldwide, and it causes disability. Thus, in individuals with post-COVID-19 syndrome, depressive symptoms and clinically severe depression may have major effects on quality of life [32]. Furthermore, after recovering from COVID-19, many people reported psychological symptoms such as stress, worry, and sadness because of the mental strain brought on by social stigma during the early phases of the outbreak. Thus, people who recovered from COVID-19 during the early pandemic, as opposed to the late epidemic, reportedly faced greater stress because of social stigma [33].
- In this study, the estimated likelihood of receiving a new diagnosis of mental disorder during the 5 years after a COVID-19 diagnosis was 7.06% for sleep disorders, 2.56% for anxiety disorders, and 3.42% for depressive disorders. Taquet et al. [20] conducted a large multicenter study that examined 62,354 confirmed patients with COVID-19 using the TriNetX analytics network (a federated network that records anonymized data from the electronic health records of 62 healthcare facilities, mostly in the United States). The study found that in comparison to six other health issues, COVID-19 was linked to a higher incidence of initial psychiatric diagnosis within 14 to 90 days following infection. The HR was highest for anxiety disorders, insomnia, and dementia. Following a COVID-19 diagnosis, the estimated likelihood of receiving a new mental health diagnosis at 90 days was 5.8%, 0.1% for psychotic disorders, 2.0% for mood disorders, 4.7% for anxiety disorders, and 1.9% for insomnia. Since Taquet et al. [20] followed up for only 90 days after COVID-19 diagnosis, it is difficult to directly compare their findings with those of the present study. Our study followed up for 5 years after COVID-19 diagnosis, and the HRs for sleep, anxiety, and depressive disorders were 2.27, 1.90, and 1.54, respectively.
- The HRs for mental disorders in patients with COVID-19 were analyzed in a previous study, and the findings were similar to those of the present study. Taquet et al. [34] compared the HRs for mental disorders (first diagnosis) in patients with COVID-19 to those with influenza and other respiratory tract infections. Relative to patients with influenza, the HRs for the first diagnosis of mental disorders in patients with COVID-19 were reported to be 1.79, 1.78, 2.16, 1.22, and 1.92 for mood disorders, anxiety disorders, psychotic disorders, substance use disorders, and insomnia, respectively. Relative to patients with other respiratory tract infections, the HRs for the first diagnosis of mental disorders in patients with COVID-19 were reported to be 1.41, 1.48, 1.82, 0.92, and 1.43 for mood disorders, anxiety disorders, psychotic disorders, substance use disorders, and insomnia, respectively. Because the clinical parameters and follow-up periods of the present study and those of Taquet et al. [34] were different, directly comparing the results of the two studies was difficult. However, the results of this and previous studies confirm that people who had COVID-19 have a higher incidence of major mental disorders than those who did not or had other respiratory tract infections.
- This study had several limitations. First, a medical record review could not verify specific clinical information because the study’s analysis relied on the CDM’s de-identified database, which safeguards patient privacy. Second, the CDM data used in this study are prone to data quality problems inherent in converting electronic medical records into CDM databases. Finally, this study was conducted on a limited number of patients who visited a university hospital, which is insufficient to investigate the incidence of various mental disorders. Thus, it was not possible to examine the incidence of other less common diseases, and it was impossible to investigate the incidence of specific disease subtypes. Therefore, further multi-institutional collaborative research is required.
- In summary, this study investigated the differences in the incidence of the first diagnosis of major mental disorders according to the presence or absence of COVID-19 among patients who visited a university hospital. The COVID-19 group had a higher incidence of dementia as well as sleep, anxiety, and depressive disorders than the control group. According to this study, the major mental disorders linked to COVID-19 are sleep disorders, anxiety disorders, depressive disorders, and dementia.
Discussion
-
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
-
Funding
None.
Article information
Fig. 1.Flowchart of study subjects in the Common Data Model network. COVID-19, coronavirus disease 2019.


Fig. 2.Observed hazard ratio and 95% confidence interval for major mental disorders between the COVID-19 and control groups. COVID-19, coronavirus disease 2019.


Fig. 3.The Kaplan-Meier survival curves for the risks of major mental disorders in COVID-19 and control groups. (A) Depressive disorders, (B) anxiety disorders, (C) sleep disorders, and (D) dementia. COVID-19, coronavirus disease 2019.


Table 1.Baseline demographic characteristics of study subjects before and after propensity score (PS) adjustment
Table 2.Baseline medical history (general) of study subjects before and after propensity score (PS) adjustment
Table 3.Baseline medical history (cardiovascular disease) of study subjects before and after propensity score (PS) adjustment
Table 4.Baseline medical history (neoplasms) of study subjects before and after propensity score (PS) adjustment
Table 5.Baseline medication use history of study subjects before and after propensity score (PS) adjustment
Table 6.Incidence rates and risk of major mental disorders between COVID-19 and control groups
- 1. Moeti M, Gao GF, Herrman H. Global pandemic perspectives: public health, mental health, and lessons for the future. Lancet 2022;400:e3–7.ArticlePubMedPMC
- 2. Torales J, O’Higgins M, Castaldelli-Maia JM, Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int J Soc Psychiatry 2020;66:317–20.ArticlePubMedPDF
- 3. Jin Y, Sun T, Zheng P, An J. Mass quarantine and mental health during COVID-19: a meta-analysis. J Affect Disord 2021;295:1335–46.ArticlePubMedPMC
- 4. Walton M, Murray E, Christian MD. Mental health care for medical staff and affiliated healthcare workers during the COVID-19 pandemic. Eur Heart J Acute Cardiovasc Care 2020;9:241–7.ArticlePubMedPDF
- 5. Brooks SK, Webster RK, Smith LE, Woodland L, Wessely S, Greenberg N, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet 2020;395:912–20.ArticlePubMedPMC
- 6. Dubey S, Biswas P, Ghosh R, Chatterjee S, Dubey MJ, Chatterjee S, et al. Psychosocial impact of COVID-19. Diabetes Metab Syndr 2020;14:779–88.ArticlePubMedPMC
- 7. Manchia M, Gathier AW, Yapici-Eser H, Schmidt MV, de Quervain D, van Amelsvoort T, et al. The impact of the prolonged COVID-19 pandemic on stress resilience and mental health: a critical review across waves. Eur Neuropsychopharmacol 2022;55:22–83.ArticlePubMed
- 8. Jeamjitvibool T, Duangchan C, Mousa A, Mahikul W. The association between resilience and psychological distress during the COVID-19 pandemic: a systematic review and meta-analysis. Int J Environ Res Public Health 2022;19:14854.ArticlePubMedPMC
- 9. Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 2020;87:18–22.ArticlePubMedPMC
- 10. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020;77:683–90.ArticlePubMed
- 11. Tavares-Júnior JW, de Souza AC, Borges JW, Oliveira DN, Siqueira-Neto JI, Sobreira-Neto MA, et al. COVID-19 associated cognitive impairment: a systematic review. Cortex 2022;152:77–97.ArticlePubMedPMC
- 12. Hripcsak G, Duke JD, Shah NH, Reich CG, Huser V, Schuemie MJ, et al. Observational Health Data Sciences and Informatics (OHDSI): opportunities for observational researchers. Stud Health Technol Inform 2015;216:574–8.PubMedPMC
- 13. FeederNet: a distributed clinical data analysis platform in Korea [Internet]. Seongnam, Korea: Evidnet; 2022 [cited 2023 Mar 20]. https://feedernet.com/member/main.
- 14. Tian Y, Schuemie MJ, Suchard MA. Evaluating large-scale propensity score performance through real-world and synthetic data experiments. Int J Epidemiol 2018;47:2005–14.ArticlePubMedPMC
- 15. Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993;46:1075–9.ArticlePubMed
- 16. Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol 2001;2:169–88.
- 17. International Health Terminology Standards Development Organisation (IHTSDO). SNOEMD clinical terms [Internet]. London: IHTSDO; 2019 [cited 2023 Mar 20]. http://www.snomed.org.
- 18. World Health Organization (WHO). International Statistical Classification of Diseases and Related Health Problems. 10th ed. Geneva: WHO; 2004.
- 19. Rassen JA, Shelat AA, Myers J, Glynn RJ, Rothman KJ, Schneeweiss S. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf 2012;21(Suppl 2):69–80.ArticlePubMedPDF
- 20. Taquet M, Luciano S, Geddes JR, Harrison PJ. Bidirectional associations between COVID-19 and psychiatric disorder: retrospective cohort studies of 62 354 COVID-19 cases in the USA. Lancet Psychiatry 2021;8:130–40.ArticlePubMed
- 21. Gollop C, Zingel R, Jacob L, Smith L, Koyanagi A, Kostev K. Incidence of newly-diagnosed dementia after COVID-19 infection versus acute upper respiratory infection: a retrospective cohort study. J Alzheimers Dis 2023;93:1033–40.ArticlePubMed
- 22. Freudenberg-Hua Y, Makhnevich A, Li W, Liu Y, Qiu M, Marziliano A, et al. Psychotropic medication use is associated with greater 1-year incidence of dementia after COVID-19 hospitalization. Front Med (Lausanne) 2022;9:841326.ArticlePubMedPMC
- 23. Taquet M, Sillett R, Zhu L, Mendel J, Camplisson I, Dercon Q, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry 2022;9:815–27.ArticlePubMedPMC
- 24. Xie Y, Xu E, Al-Aly Z. Risks of mental health outcomes in people with COVID-19: cohort study. BMJ 2022;376:e068993.ArticlePubMed
- 25. Hosp JA, Dressing A, Blazhenets G, Bormann T, Rau A, Schwabenland M, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain 2021;144:1263–76.ArticlePubMedPDF
- 26. Spudich S, Nath A. Nervous system consequences of COVID-19. Science 2022;375:267–9.ArticlePubMed
- 27. Lyra E Silva NM, Barros-Aragão FG, De Felice FG, Ferreira ST. Inflammation at the crossroads of COVID-19, cognitive deficits and depression. Neuropharmacology 2022;209:109023.ArticlePubMedPMC
- 28. Bhat S, Chokroverty S. Sleep disorders and COVID-19. Sleep Med 2022;91:253–61.ArticlePubMed
- 29. Tedjasukmana R, Budikayanti A, Islamiyah WR, Witjaksono AM, Hakim M. Sleep disturbance in post COVID-19 conditions: prevalence and quality of life. Front Neurol 2023;13:1095606.ArticlePubMedPMC
- 30. Witthauer C, Gloster AT, Meyer AH, Goodwin RD, Lieb R. Comorbidity of infectious diseases and anxiety disorders in adults and its association with quality of life: a community study. Front Public Health 2014;2:80.ArticlePubMedPMC
- 31. Fardin MA. COVID-19 and anxiety: a review of psychological impacts of infectious disease outbreaks. Arch Clin Infect Dis 2020;15(COVID-19):e102779.Article
- 32. Mazza MG, Palladini M, Poletti S, Benedetti F. Post-COVID-19 depressive symptoms: epidemiology, pathophysiology, and pharmacological treatment. CNS Drugs 2022;36:681–702.ArticlePubMedPMCPDF
- 33. Kim D. Post-recovery stigma in early and late COVID-19 epidemic. Keimyung Med J 2022;41:80–3.ArticlePDF
- 34. Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry 2021;8:416–27.ArticlePubMedPMC
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Figure
- Related articles
-
- Optimal examination for traumatic nerve/muscle injuries in earthquake survivors: a retrospective observational study
- Incidence of congenital hypothyroidism by gestational age: a retrospective observational study
- The impact of quality of life measured by WHOQOL-BREF on mortality in maintenance hemodialysis patients: a single center retrospective cross-sectional study
- Clinical investigation on acute pyelonephritis without pyuria: a retrospective observational study

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite