Indexed in: ESCI, Scopus, PubMed,
PubMed Central, CAS, DOAJ, KCI
PubMed Central, CAS, DOAJ, KCI
FREE article processing charge

Articles
- Page Path
- HOME > J Yeungnam Med Sci > Volume 39(3); 2022 > Article
-
Focused Review article
Pain in amyotrophic lateral sclerosis: a narrative review -
Soyoung Kwak

-
Journal of Yeungnam Medical Science 2022;39(3):181-189.
DOI: https://doi.org/10.12701/jyms.2022.00332
Published online: June 8, 2022
Department of Physical Medicine and Rehabilitation, Yeungnam University College of Medicine, Daegu, Korea
- Corresponding author: Soyoung Kwak, MD, MPH, PhD Department of Physical Medicine and Rehabilitation, Yeungnam University College of Medicine, 170 Hyeonchung-ro, Nam-gu, Daegu 42415, Korea Tel: +82-53-620-3270 • E-mail: sk315@ynu.ac.kr
• Received: May 10, 2022 • Revised: May 21, 2022 • Accepted: May 26, 2022
Copyright © 2022 Yeungnam University College of Medicine, Yeungnam University Institute of Medical Science
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
- Amyotrophic lateral sclerosis (ALS) is a rapidly progressive neurodegenerative condition characterized by loss of motor neurons, resulting in motor weakness of the limbs and/or bulbar muscles. Pain is a prevalent but neglected symptom of ALS, and it has a significant negative impact on the quality of life of patients and their caregivers. This review outlines the epidemiology, clinical characteristics, underlying mechanisms, and management strategies of pain in ALS to improve clinical practice and patient outcomes related to pain. Pain is a prevalent symptom among patients with ALS, with a variable reported prevalence. It may occur at any stage of the disease and can involve any part of the body without a specific pattern. Primary pain includes neuropathic pain and pain from spasticity or cramps, while secondary pain is mainly nociceptive, occurring with the progression of muscle weakness and atrophy, prolonged immobility causing degenerative changes in joints and connective tissue, and long-term home mechanical ventilation. Prior to treatment, the exact patterns and causes of pain must first be identified, and the treatment should be tailored to each patient. Treatment options can be classified into pharmacological treatments, including nonsteroidal anti-inflammatory drugs, antiepileptic drugs, drugs for cramps or spasticity, and opioid; and nonpharmacological treatments, including positioning, splints, joint injections, and physical therapy. The development of standardized and specific assessment tools for pain-specific to ALS is required, as are further studies on treatments to reduce pain, diminish suffering, and improve the quality of life of patients with ALS.
- Amyotrophic lateral sclerosis (ALS) is a rapidly progressive neurodegenerative condition characterized by loss of motor neurons in the cerebral motor cortex, brainstem, and spinal cord. It is typically heralded by motor weakness in the limbs and/or bulbar muscles and results in progressive muscle weakness beyond the site of initial symptoms, resulting in a fatal outcome generally within 2 to 4 years from the onset of disease [1]. Diagnosis of the disease is based on clinical and electrophysiological evidence of upper motor neuron (UMN) and lower motor neuron (LMN) involvement and the distribution of clinical signs of the disease (e.g., craniobulbar, cervical, thoracic, and lumbosacral regions) [2-4]. Notably, ALS should be considered a syndrome with a broad spectrum rather than a single disease entity because its symptoms and clinical course are very diverse (e.g., UMN vs. LMN predominance, bulbar vs. limb [spinal] predominance, rapid vs. slow progression, and the extent of concomitant cognitive impairment) [5]. ALS was once considered a pure motor disorder with ‘3 Ps’ (progressive, painless, and paralysis); however, a growing body of evidence indicates that ALS is a multisystem disease with early and diverse non-motor symptoms including cognitive and behavioral changes, neuropsychiatric disturbances, sleep disruption, excess secretions, metabolic abnormalities, bowel and bladder dysfunction, changes in bone health, olfactory and somatosensory impairment, and pain [6]. These non-motor symptoms of ALS have attracted research attention over the last decade, with the identification of a repeat expansion of the C9orf72 gene in some patients and many shared pathologies across the spectrum of ALS and frontotemporal dementia in these patients [7,8].
- The active study of pain in ALS, as well as other non-motor symptoms, began approximately 10 years ago, although there were earlier observations [9-11]. Previous studies revealed that these symptoms are prevalent among patients with ALS and negatively impact the lives of these patients and their caregivers, physically, psychologically, socially, and spiritually [12-15]. In addition, the two major guidelines on the management of ALS (from the American Academy of Neurology [AAN] and European Federation of Neurological Societies [EFNS]) include the treatment of pain in these patients [16,17], and there has been a Cochrane review on this subject [18]. However, a more thorough understanding of the prevalence, clinical characteristics, underlying mechanisms, and effective management of pain in this population is required to improve clinical practice and patient outcomes. In this review, the epidemiology of pain in patients with ALS, its clinical characteristics, and suggested underlying mechanisms of pain will be discussed before summarizing the assessment and treatment strategies for pain in ALS.
Introduction
- The prevalence of pain in patients with ALS varies greatly from as low as 15% to as high as 85% [10,13,15,19-24]. These differences are thought to arise from the different study designs and settings, definitions of pain, and tools used to assess pain. A recent meta-analysis on the prevalence of pain in ALS estimated the pooled prevalence to be 60% (95% confidence interval [CI], 50%–69%) with a high degree of heterogeneity (I2=94%, p<0.001) [25]. This meta-analysis also found that studies using tailored measures (e.g., clinical interviews, unvalidated questionnaires, or retrospective reviews of the medical record) to assess pain tended to show a lower pooled prevalence of 47% (95% CI, 34%–60%) and greater heterogeneity (I2=90%, p<0.001) compared to those using tailored measures (I2=82%, p=0.002, respectively), while studies using only validated measures showed a pooled pain prevalence of 65% (95% CI, 56%–73%, similar to the overall pooled prevalence of 60%) and lower heterogeneity. This demonstrates the importance of appropriate assessment tools for pain. Nevertheless, the two major guidelines for the management of ALS do not suggest which assessment tools should be selected. Another study showed that less than 20% of ALS clinics used questionnaires involving pain rating scales other than just open-ended questions [19]. Therefore, the establishment of a standardized assessment tool for pain in ALS is necessary to better manage pain in patients with ALS.
- Currently, the most frequently used formal assessment tool is the Brief Pain Inventory questionnaire [13,15,19,22,24], which assesses current pain, its intensity over the previous week, and the extent of interference with mood, sleep, and physical, working, and social activities. Other validated assessment tools include the Neuropathic Pain Scale, short-form McGill Pain Questionnaire, Neuropathic Pain Symptom Inventory, and Neuropathic Pain Diagnostic Questionnaire [20,23]. However, although these tools are validated and are useful in discriminating and describing pain, it is unclear how useful they are for assessing pain in patients with ALS, who often have more than one type of pain [26]. Therefore, the development of standardized assessment tools for the diagnosis and monitoring of pain in ALS is important for improving patient care and outcomes.
- In addition, the attention that physicians and patients pay to pain might also result in an inconsistency in its reported prevalence [26]. For example, a recent study conducted by Åkerblom et al. [12] indicated that some patients with ALS who were experiencing pain did not report it to their physician because they thought pain was not as important as other symptoms of the disease, they had received insufficient attention from the physician, or they believed there was no effective pain treatment, just as there was no effective disease treatment. Considering these points, it is important not only to evaluate pain at every visit using a structured assessment tool but also to train healthcare professionals accordingly.
Epidemiology of pain in amyotrophic lateral sclerosis
- Primary pain in patients with ALS includes neuropathic pain and pain from spasticity or cramps. Secondary pain is mainly nociceptive, occurring with the progression of muscle weakness and atrophy, prolonged immobility causing degenerative changes in joints and connective tissue, and long-term home mechanical ventilation (HMV) either noninvasively by mask interface or invasively by tracheostomy (Fig. 1) [26,27].
- Pain in ALS is largely nociceptive in nature, although it cannot explain all types of ALS pain. Neuropathic pain has not been reported to be prevalent among patients with ALS [16,26,28-30]. Previous studies have indicated a prevalence of neuropathic pain of 9% [28], 1% [29], and 5% [30] using Douleur Neuropathique 4 (DN4) as a screening tool; these values are not higher than those of the general population, ranging from 6.9% to 10.0%, as reported in a previous systematic review [31]. However, a recent study indicated that 62.5% of patients with ALS experienced neuropathic pain, described as electric shock, burning, dull, stabbing, throbbing, painful cold, sharp, or shooting [32]. However, that study did not adopt a validated measure for neuropathic pain. Although neuropathic pain seems to be rare in ALS, this finding might result from the fact that DN4 is only a screening tool for neuropathic pain and may not be a definite measure to diagnose neuropathic pain in ALS [28]. Furthermore, the low prevalence of neuropathic pain might have occurred because a large number of patients were already taking neurotropic medications such as riluzole, the only drug approved by the U.S. Food and Drug Administration for ALS treatment, which blocks the presynaptic release of glutamate [33] and thereby might reduce neuropathic pain [29,34,35]. Some neuropathic pain might be due to spinal disorders, at least partially, considering that the peak incidence of ALS occurs between 58 and 63 years of age [1], an age group in which spinal disorders are common. Although it has been reported that 14% to 32% of the diagnostic delay in ALS is due to the misdiagnosis of ALS as a spinal disorder such as myelopathy or radiculopathy [36-42], no study has evaluated the exact prevalence of spinal disorders in patients with ALS.
- Other sources of primary pain include cramps and spasticity. Muscle cramp refers to a sudden and involuntary muscle contraction originating from the peripheral nerves [43] and is reported to be a major source of pain in patients with ALS, affecting approximately two-thirds of patients [11,44]. In a previous study, which followed 41 patients with ALS for up to 21 months, 95% of the patients had experienced cramps over the course of the disease [45]. Muscle cramps in patients with ALS are believed to originate from instability of the affected motor unit and are typically associated with denervation of the muscles [43]; patients with limb-onset ALS are more frequently affected by them [43,45]. Spasticity is a velocity-dependent increase in muscle tone, which results from the loss of inhibitory control of UMNs, causing stiffness and spasms in the affected muscles as well as difficulties in fine motor control [46]. According to a recent study conducted in a tertiary hospital in France, the prevalence of spasticity was 36% in patients with ALS, and approximately half (42.5%) of those patients had mild pain. However, 16.7% of patients with spasticity had moderate to severe pain, with numeric rating scale scores of ≥4 [47].
- Secondary pain is mainly nociceptive, which means it arises from nonneuronal tissue damage or the activation of peripheral nociceptors as a result of mechanical or other noxious stimuli [48]. Secondary pain is known to develop as ALS progresses. Joint pain, which is prevalent in patients with ALS, develops when weakened and wasting muscles are unable to provide support to the joint [26,49]. It has been reported that shoulders and hips are the most frequently affected joints [13,50]. Immobility might cause skin pressure, which might be perceived as pain, although pressure sores do not occur frequently despite the poor mobility of patients with ALS [51]. Long-term HMV may be another source of pain. In patients undergoing noninvasive ventilation (NIV), skin problems due to mask interfaces, especially on the nasal bridge, can cause pain [26]. In invasively ventilated patients with a tracheostomy who remain in the same position over a period of time during HMV, irritation of the throat from the weight of the circuits (tubes) and suctioning of the secretions can result in significant discomfort and pain, which are often unnoticed by caregivers or medical professionals [52].
- In terms of the localization of pain, previous studies have not found a specific pattern, and pain can involve any part of the body, including the proximal and distal sites of the upper and lower extremities and the back, or it could be diffuse [13,15,19,22,23,53]. A recent meta-analysis, which analyzed a total of 393 patients with ALS who reported 715 locations of pain, reported that the most commonly reported location was the upper limbs (including shoulders and extremities) (41.5%), followed by the lower limbs (33.7%), and the head, neck, trunk, and back (24.8%) [25].
- The intensity of pain was also reported in the same study; among 1,426 patients who reported pain intensity, 78.8% reported moderate pain, 17.5% reported severe pain, and only 2.0% and 1.7% reported mild and very severe pain, respectively [25], which is consistent with previous reports [23,29]. However, other studies have indicated that the intensity was mainly mild [14,22]. Again, this inconsistency might result from the different study designs and settings, the definition of pain, and the assessment tools for pain, as well as the cross-sectional nature of the aforementioned studies. A recent longitudinal study indicated that at least moderate average pain was reported in about two-thirds of patients who completed a 1-year follow-up [54], indicating that moderate to severe pain might be persistent in patients with ALS.
- Several studies have indicated the occurrence of pain early in the disease course [9,15,19,26,32,55]. In a study including 424 patients, 34% of patients with ALS reported pain in the early stage of disease [19], and a recent epidemiological study revealed that patients with ALS had been prescribed more drugs for neuropathic pain (hazard ratio, 1.84; 95% CI, 0.99–3.42) up to 2 years before the onset of ALS [56]. The question of whether pain worsens with ALS progression varies widely among studies [13-15,22,23,53], probably because of the cross-sectional nature of these studies [26]. A longitudinal study indicated that pain intensity increased by 1 point on the visual analog scale from the first visit to the last, with a median follow-up period of 104 days (range, 35–846 days) [57]. Similarly, a recent study including 151 patients at baseline also revealed that the intensity and quality of pain and the impairment it causes did not change significantly over time [54], which is consistent with findings from another longitudinal study [58].
Characteristics of pain
- Currently, there is little high-quality evidence regarding the pharmacological treatment of pain in ALS, as there are no randomized or quasi-randomized treatment trials of pain in ALS. However, several guidelines on the clinical management of ALS include management of pain, cramps, and spasticity [16,17,59], and there are Cochrane reviews [18,60]. Prior to treatment, the exact patterns and causes of pain must first be identified; then, the treatment should be tailored to each patient. Treatment options can be classified into pharmacological and nonpharmacological treatments, for which pharmacological treatment is known to be more effective for the primary pain, whereas nonpharmacological approaches are regarded as more effective for secondary pain in ALS [26]. A summary of interventions for the management of pain according to its etiology is presented in Table 1, including both self-treatment and professional treatment.
- 1. Treatment of primary pain in amyotrophic lateral sclerosis
- As stated earlier, neuropathic pain does not appear to be prevalent in patients with ALS; however, this type of pain is significant because it can be controlled according to pharmacological management pathways for neuropathic pain [61]. The most widely used drugs for the treatment of neuropathic pain are gabapentin, pregabalin, and tricyclic antidepressants [49]. Although opioids are not recommended as first-line therapy, they can be used when pain is not controlled or in advanced stages in cases of increased pain or with symptoms related to respiratory insufficiency, such as dyspnea and sleep disturbance [62]. However, there is little evidence to support the safety and efficacy of opioid use [18].
- Quinine sulfate is the most frequently prescribed drug in European countries, although its use for cramps is proscribed by the U.S. Food and Drug Administration [26,63]. It should also be noted that quinine sulfate can cause serious side effects, such as thrombocytopenia and drug interactions [49]. Mexiletine at a dose of 300 mg per day and dronabinol at a dose of 5 mg twice daily could be used as first-line therapies; gabapentin and levetiracetam are suggested as second-line therapies in the guidelines [16,17].
- Spasticity can be effectively managed with appropriate medication. Although there are no controlled clinical trials in patients with ALS, baclofen, tizanidine, benzodiazepines, dantrolene, and carbamazepine can be used to reduce spasticity [49]. However, undesirable side effects, such as weakness, daytime somnolence, or excessive fatigue, are common with these medications. Therefore, careful dose titration and appropriate physiotherapy are required [50,64,65]. In cases of refractory spasticity, intrathecal baclofen pump placement was proposed as an option, and a previous study indicated an average reduction of 54% in pain intensity after the procedure. However, these findings should be interpreted with caution considering the open-label study design and lack of follow-up evaluation [66].
- 2. Treatment of secondary pain in amyotrophic lateral sclerosis
- Musculoskeletal pain secondary to progressive wasting and weakness of the muscles should be managed with careful positioning, regular gentle range of movement exercises, joint injections, and medications [11,26]. The timely and proper use of assistive devices is also an important point of care, which includes the use of special mattresses and pillows, custom-fitted wheelchairs, and neutral-position splints for the hands and ankles to prevent joint contractures [64,65].
- If pain is not controlled by positioning, splints, joint injections, or physical therapy, regular administration of analgesics should be considered using the World Health Organization analgesic ladder originally developed for the management of cancer-related pain, as suggested by the AAN Practice Parameters and EFNS guidelines [16,17]. However, the use of this strategy in patients with ALS is still debatable [67,68].
Treatment of pain in amyotrophic lateral sclerosis
- Pain is an important issue in ALS, with a pronounced impact on quality of life and suffering. The ultimate goal of pain treatment is to reduce its intensity, diminish suffering, and improve the quality of life of patients. To achieve this goal, thorough and timely assessments are vital. However, there is currently no assessment tool specific to this population. Therefore, a consensus should be reached regarding the timing, intervals, and contents of pain assessments in ALS. In addition, specific guidelines on the treatment of pain in ALS should be developed because successful pain treatment can improve the quality of life of patients and their caregivers, even in the absence of a disease cure. Further research on this topic is needed, considering the gaps between current care standards and patient requirements. In particular, careful consideration of the study design is required [26], considering that a classic double-blind, placebo-controlled study might not be feasible owing to the nature of the disease (rare, rapidly progressing, and incurable). Until more robust evidence is available, strategies for managing pain in ALS should be tailored to the needs of individual patients, based on good clinical practices and information from the management of nonmalignant chronic pain.
Conclusion
-
Conflicts of interest
No potential conflicts of interest relevant to this article was reported.
-
Funding
None.
Notes
Fig. 1.Types of pain in amyotrophic lateral sclerosis. Most reported types of pain are secondary in nature (mainly nociceptive; blue shading), but there is some evidence for primary forms of pain (green shading), such as neuropathic pain, spasticity, and cramps. NIV, noninvasive ventilation. Reprinted from Chiò et al. [26] with permission from Elsevier.
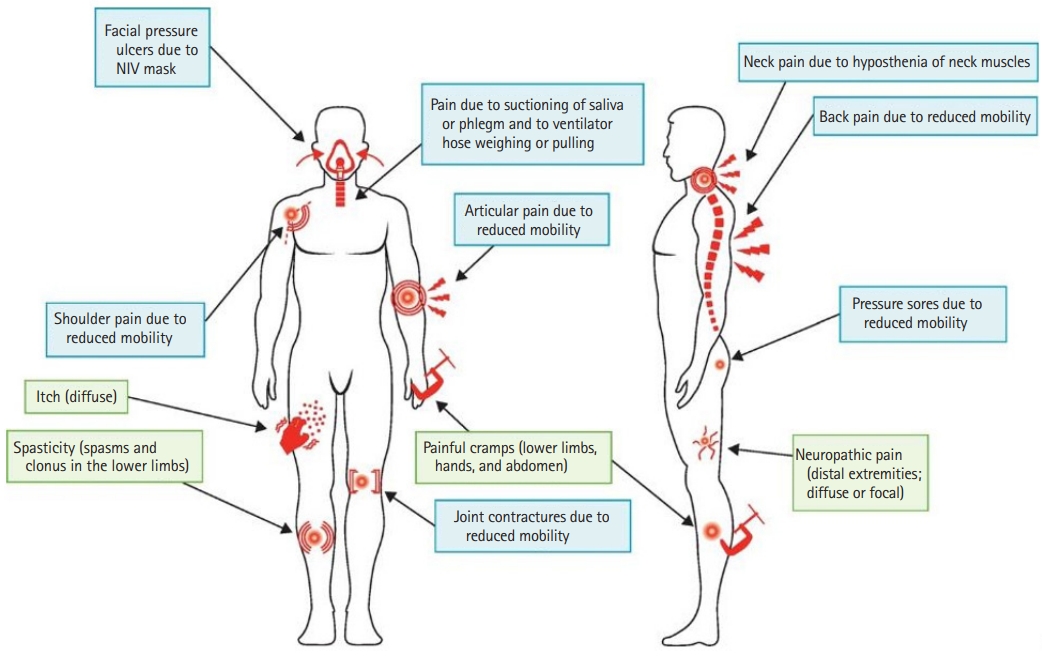

Table 1.Interventions for the management of pain in patients with amyotrophic lateral sclerosis according to the etiology of pain
- 1. Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet 2011;377:942–55.ArticlePubMed
- 2. Brooks BR. El Escorial World Federation of Neurology criteria for the diagnosis of amyotrophic lateral sclerosis. J Neurol Sci 1994;124(Suppl):96–107.ArticlePubMed
- 3. Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–9.ArticlePubMed
- 4. de Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol 2008;119:497–503.
- 5. Turner M, Jenkins L. Defining the syndrome. In: Turner M, Jenkins L, editors. Diagnosing amyotrophic lateral sclerosis: clinical wisdom to facilitate faster diagnosis. Oxford: S. Karger Publishers Limited; 2020. p. 7–24.ArticlePubMedPDF
- 6. Mahoney CJ, Ahmed RM, Huynh W, Tu S, Rohrer JD, Bedlack RS, et al. Pathophysiology and treatment of non-motor dysfunction in amyotrophic lateral sclerosis. CNS Drugs 2021;35:483–505.ArticlePubMedPMC
- 7. Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron 2011;72:257–68.ArticlePubMed
- 8. Burrell JR, Kiernan MC, Vucic S, Hodges JR. Motor neuron dysfunction in frontotemporal dementia. Brain 2011;134(Pt 9):2582–94.ArticlePubMed
- 9. Drake ME Jr. Chronic pain syndrome in amyotrophic lateral sclerosis. Arch Neurol 1983;40:453–4.ArticlePubMed
- 10. de Castro-Costa CM, Oriá RB, Machado-Filho JA, Franco MT, Diniz DL, Giffoni SD, et al. Amyotrophic lateral sclerosis: clinical analysis of 78 cases from Fortaleza (northeastern Brazil). Arq Neuropsiquiatr 1999;57(3B):761–74.ArticlePubMed
- 11. Ganzini L, Johnston WS, Hoffman WF. Correlates of suffering in amyotrophic lateral sclerosis. Neurology 1999;52:1434–40.ArticlePubMed
- 12. Åkerblom Y, Jakobsson Larsson B, Zetterberg L, Åsenlöf P. The multiple faces of pain in motor neuron disease: a qualitative study to inform pain assessment and pain management. Disabil Rehabil 2020;42:2123–32.ArticlePubMed
- 13. Chiò A, Canosa A, Gallo S, Moglia C, Ilardi A, Cammarosano S, et al. Pain in amyotrophic lateral sclerosis: a population-based controlled study. Eur J Neurol 2012;19:551–5.ArticlePubMedPMC
- 14. Raheja D, Stephens HE, Lehman E, Walsh S, Yang C, Simmons Z. Patient-reported problematic symptoms in an ALS treatment trial. Amyotroph Lateral Scler Frontotemporal Degener 2016;17:198–205.ArticlePubMed
- 15. Wallace VC, Ellis CM, Burman R, Knights C, Shaw CE, Al-Chalabi A. The evaluation of pain in amyotrophic lateral sclerosis: a case controlled observational study. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:520–7.ArticlePubMed
- 16. EFNS Task Force on Diagnosis and Management of Amyotrophic Lateral Sclerosis; Andersen PM, Abrahams S, Borasio GD, de Carvalho M, Chio A, et al. EFNS guidelines on the clinical management of amyotrophic lateral sclerosis (MALS): revised report of an EFNS task force. Eur J Neurol 2012;19:360–75.ArticlePubMedPMC
- 17. Miller RG, Jackson CE, Kasarskis EJ, England JD, Forshew D, Johnston W, et al. Practice parameter update: the care of the patient with amyotrophic lateral sclerosis: multidisciplinary care, symptom management, and cognitive/behavioral impairment (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2009;73:1227–33.ArticlePubMedPMC
- 18. Brettschneider J, Kurent J, Ludolph A. Drug therapy for pain in amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst Rev 2013;2013:CD005226.ArticlePubMed
- 19. Stephens HE, Lehman E, Raheja D, Yang C, Walsh S, Mcarthur DB, et al. Pain in amyotrophic lateral sclerosis: patient and physician perspectives and practices. Amyotroph Lateral Scler Frontotemporal Degener 2015;17:21–9.ArticlePubMedPDF
- 20. Truini A, Biasiotta A, Onesti E, Di Stefano G, Ceccanti M, La Cesa S, et al. Small-fibre neuropathy related to bulbar and spinal-onset in patients with ALS. J Neurol 2015;262:1014–8.ArticlePubMed
- 21. Dalla Bella E, Lombardi R, Porretta-Serapiglia C, Ciano C, Gellera C, Pensato V, et al. Amyotrophic lateral sclerosis causes small fiber pathology. Eur J Neurol 2016;23:416–20.ArticlePubMedPMCPDF
- 22. Hanisch F, Skudlarek A, Berndt J, Kornhuber ME. Characteristics of pain in amyotrophic lateral sclerosis. Brain Behav 2015;5:e00296.ArticlePubMedPMC
- 23. Pizzimenti A, Aragona M, Onesti E, Inghilleri M. Depression, pain and quality of life in patients with amyotrophic lateral sclerosis: a cross-sectional study. Funct Neurol 2013;28:115–9.ArticlePubMed
- 24. Jensen MP, Abresch RT, Carter GT, McDonald CM. Chronic pain in persons with neuromuscular disease. Arch Phys Med Rehabil 2005;86:1155–63.ArticlePubMed
- 25. Hurwitz N, Radakovic R, Boyce E, Peryer G. Prevalence of pain in amyotrophic lateral sclerosis: a systematic review and meta-analysis. Amyotroph Lateral Scler Frontotemporal Degener 2021;22:449–58.ArticlePubMed
- 26. Chiò A, Mora G, Lauria G. Pain in amyotrophic lateral sclerosis. Lancet Neurol 2017;16:144–57.ArticlePubMed
- 27. Borasio GD, Voltz R, Miller RG. Palliative care in amyotrophic lateral sclerosis. Neurol Clin 2001;19:829–47.ArticlePubMedPDF
- 28. Moisset X, Cornut-Chauvinc C, Clavelou P, Pereira B, Dallel R, Guy N. Is there pain with neuropathic characteristics in patients with amyotrophic lateral sclerosis?: a cross-sectional study. Palliat Med 2016;30:486–94.ArticlePubMedPDF
- 29. Lopes LC, Galhardoni R, Silva V, Jorge FM, Yeng LT, Callegaro D, et al. Beyond weakness: Characterization of pain, sensory profile and conditioned pain modulation in patients with motor neuron disease: a controlled study. Eur J Pain 2018;22:72–83.ArticlePubMedPMCPDF
- 30. An R, Li Y, He X, Li C, Li X, Xu Y, et al. The evaluation of pain with nociceptive and neuropathic characteristics from three different perspectives in amyotrophic lateral sclerosis patients: a case controlled observational study in Southwestern China. Neural Plast 2021;2021:5537892.ArticlePubMed
- 31. van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain 2014;155:654–62.ArticlePubMed
- 32. Taga A, Schito P, Trapasso MC, Zinno L, Pavesi G. Pain at the onset of amyotrophic lateral sclerosis: a cross-sectional study. Clin Neurol Neurosurg 2019;186:105540.ArticlePubMedPMC
- 33. Miller RG, Mitchell JD, Moore DH. Riluzole for amyotrophic lateral sclerosis (ALS)/motor neuron disease (MND). Cochrane Database Syst Rev 2012;2012:CD001447.ArticlePubMed
- 34. Moon ES, Karadimas SK, Yu WR, Austin JW, Fehlings MG. Riluzole attenuates neuropathic pain and enhances functional recovery in a rodent model of cervical spondylotic myelopathy. Neurobiol Dis 2014;62:394–406.ArticlePubMed
- 35. Poupon L, Lamoine S, Pereira V, Barriere DA, Lolignier S, Giraudet F, et al. Targeting the TREK-1 potassium channel via riluzole to eliminate the neuropathic and depressive-like effects of oxaliplatin. Neuropharmacology 2018;140:43–61.ArticlePubMed
- 36. Palese F, Sartori A, Logroscino G, Pisa FE. Predictors of diagnostic delay in amyotrophic lateral sclerosis: a cohort study based on administrative and electronic medical records data. Amyotroph Lateral Scler Frontotemporal Degener 2019;20:176–85.ArticlePubMedPMC
- 37. Galvin M, Ryan P, Maguire S, Heverin M, Madden C, Vajda A, et al. The path to specialist multidisciplinary care in amyotrophic lateral sclerosis: a population- based study of consultations, interventions and costs. PLoS One 2017;12:e0179796.ArticlePubMedPMC
- 38. Paganoni S, Macklin EA, Lee A, Murphy A, Chang J, Zipf A, et al. Diagnostic timelines and delays in diagnosing amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler Frontotemporal Degener 2014;15:453–6.ArticlePubMed
- 39. Belsh JM, Schiffman PL. Misdiagnosis in patients with amyotrophic lateral sclerosis. Arch Intern Med 1990;150:2301–5.ArticlePubMed
- 40. Chiò A. ISIS Survey: an international study on the diagnostic process and its implications in amyotrophic lateral sclerosis. J Neurol 1999;246(Suppl 3):III1–5.ArticlePubMed
- 41. Cellura E, Spataro R, Taiello AC, La Bella V. Factors affecting the diagnostic delay in amyotrophic lateral sclerosis. Clin Neurol Neurosurg 2012;114:550–4.ArticlePubMed
- 42. Househam E, Swash M. Diagnostic delay in amyotrophic lateral sclerosis: what scope for improvement? J Neurol Sci 2000;180:76–81.ArticlePubMed
- 43. Miller TM, Layzer RB. Muscle cramps. Muscle Nerve 2005;32:431–42.
- 44. Swash M, Czesnik D, de Carvalho M. Muscular cramp: causes and management. Eur J Neurol 2019;26:214–21.ArticlePubMedPDF
- 45. Caress JB, Ciarlone SL, Sullivan EA, Griffin LP, Cartwright MS. Natural history of muscle cramps in amyotrophic lateral sclerosis. Muscle Nerve 2016;53:513–7.ArticlePubMedPMC
- 46. Mayer NH. Clinicophysiologic concepts of spasticity and motor dysfunction in adults with an upper motoneuron lesion. Muscle Nerve Suppl 1997;6:S1–13.ArticlePubMed
- 47. Verschueren A, Grapperon AM, Delmont E, Attarian S. Prevalence of spasticity and spasticity-related pain among patients with amyotrophic lateral sclerosis. Rev Neurol (Paris) 2021;177:694–8.ArticlePubMed
- 48. Smart KM, Blake C, Staines A, Doody C. Clinical indicators of ‘nociceptive’, ‘peripheral neuropathic’ and ‘central’ mechanisms of musculoskeletal pain: a Delphi survey of expert clinicians. Man Ther 2010;15:80–7.ArticlePubMed
- 49. Borasio GD, Shaw PJ, Hardiman O, Ludolph AC, Sales Luis ML, Silani V, et al. Standards of palliative care for patients with amyotrophic lateral sclerosis: results of a European survey. Amyotroph Lateral Scler Other Motor Neuron Disord 2001;2:159–64.ArticlePubMed
- 50. Ho DT, Ruthazer R, Russell JA. Shoulder pain in amyotrophic lateral sclerosis. J Clin Neuromuscul Dis 2011;13:53–5.ArticlePubMed
- 51. Hayashi T, Narita Y, Okugawa N, Hamaguchi E, Shibahara M, Kuzuhara S. Pressure ulcers in ALS patients on admission at a university hospital in Japan. Amyotroph Lateral Scler 2007;8:310–3.ArticlePubMed
- 52. Hirano YM, Yamazaki Y, Shimizu J, Togari T, Bryce TJ. Ventilator dependence and expressions of need: a study of patients with amyotrophic lateral sclerosis in Japan. Soc Sci Med 2006;62:1403–13.ArticlePubMed
- 53. Rivera I, Ajroud-Driss S, Casey P, Heller S, Allen J, Siddique T, et al. Prevalence and characteristics of pain in early and late stages of ALS. Amyotroph Lateral Scler Frontotemporal Degener 2013;14:369–72.ArticlePubMed
- 54. Wigand B, Schlichte I, Schreiber S, Heitmann J, Meyer T, Dengler R, et al. Characteristics of pain and the burden it causes in patients with amyotrophic lateral sclerosis: a longitudinal study. Amyotroph Lateral Scler Frontotemporal Degener 2022;23:284–91.ArticlePubMed
- 55. Isaacs JD, Dean AF, Shaw CE, Al-Chalabi A, Mills KR, Leigh PN. Amyotrophic lateral sclerosis with sensory neuropathy: part of a multisystem disorder? J Neurol Neurosurg Psychiatry 2007;78:750–3.ArticlePubMedPMC
- 56. D’Ovidio F, d’Errico A, Farina E, Calvo A, Costa G, Chiò A. Amyotrophic lateral sclerosis incidence and previous prescriptions of drugs for the nervous system. Neuroepidemiology 2016;47:59–66.ArticlePubMedPDF
- 57. Adelman EE, Albert SM, Rabkin JG, Del Bene ML, Tider T, O’Sullivan I. Disparities in perceptions of distress and burden in ALS patients and family caregivers. Neurology 2004;62:1766–70.ArticlePubMed
- 58. Pagnini F, Lunetta C, Banfi P, Rossi G, Fossati F, Marconi A, et al. Pain in amyotrophic lateral sclerosis: a psychological perspective. Neurol Sci 2012;33:1193–6.ArticlePubMedPDF
- 59. National Institute for Health and Care Excellence (NICE). NICE guideline. Motor neuron disease: assessment and management [Internet]. London: NICE; 2016 [cited 2022 May 8]. https://www.nice.org.uk/guidance/ng42.
- 60. Ng L, Khan F, Young CA, Galea M. Symptomatic treatments for amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst Rev 2017;1:CD011776.ArticlePubMedPMC
- 61. Cruccu G, Truini A. A review of neuropathic pain: from guidelines to clinical practice. Pain Ther 2017;6(Suppl 1):35–42.ArticlePubMedPMCPDF
- 62. Oliver D. Opioid medication in the palliative care of motor neurone disease. Palliat Med 1998;12:113–5.ArticlePubMedPDF
- 63. Baldinger R, Katzberg HD, Weber M. Treatment for cramps in amyotrophic lateral sclerosis/motor neuron disease. Cochrane Database Syst R 2012;(4):CD004157.Article
- 64. Bello-Haas VD. Physical therapy for individuals with amyotrophic lateral sclerosis: current insights. Degener Neurol Neuromuscul Dis 2018;8:45–54.ArticlePubMedPMC
- 65. Majmudar S, Wu J, Paganoni S. Rehabilitation in amyotrophic lateral sclerosis: why it matters. Muscle Nerve 2014;50:4–13.ArticlePubMedPMCPDF
- 66. McClelland S 3rd, Bethoux FA, Boulis NM, Sutliff MH, Stough DK, Schwetz KM, et al. Intrathecal baclofen for spasticity-related pain in amyotrophic lateral sclerosis: efficacy and factors associated with pain relief. Muscle Nerve 2008;37:396–8.ArticlePubMed
- 67. Yang J, Bauer BA, Wahner-Roedler DL, Chon TY, Xiao L. The modified WHO analgesic ladder: is it appropriate for chronic non-cancer pain? J Pain Res 2020;13:411–7.ArticlePubMedPMC
- 68. Anekar AA, Cascella M. WHO analgesic ladder. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 [cited 2022 May 10]. https://www.ncbi.nlm.nih.gov/books/NBK554435/.
References
Figure & Data
References
Citations
Citations to this article as recorded by 

- Health-related quality of life across disease stages in patients with amyotrophic lateral sclerosis: results from a real-world survey
Katie Stenson, T. E. Fecteau, L. O’Callaghan, P. Bryden, J. Mellor, J. Wright, L. Earl, O. Thomas, H. Iqbal, S. Barlow, S. Parvanta
Journal of Neurology.2024; 271(5): 2390. CrossRef - Amyotrophic Lateral Sclerosis and Pain: A Narrative Review from Pain Assessment to Therapy
Vincenzo Pota, Pasquale Sansone, Sara De Sarno, Caterina Aurilio, Francesco Coppolino, Manlio Barbarisi, Francesco Barbato, Marco Fiore, Gianluigi Cosenza, Maria Beatrice Passavanti, Maria Caterina Pace, Enzo Emanuele
Behavioural Neurology.2024; 2024: 1. CrossRef - Non-motor symptoms in patients with amyotrophic lateral sclerosis: current state and future directions
Bogdan Bjelica, Maj-Britt Bartels, Jasper Hesebeck-Brinckmann, Susanne Petri
Journal of Neurology.2024; 271(7): 3953. CrossRef - Likely Pathogenic Variants of Cav1.3 and Nav1.1 Encoding Genes in Amyotrophic Lateral Sclerosis Could Elucidate the Dysregulated Pain Pathways
Zsófia Flóra Nagy, Balázs Sonkodi, Margit Pál, Péter Klivényi, Márta Széll
Biomedicines.2023; 11(3): 933. CrossRef - Palliative Care in Amyotrophic Lateral Sclerosis
Sebastiano Mercadante, Lou'i Al-Husinat
Journal of Pain and Symptom Management.2023; 66(4): e485. CrossRef - The blind spot and challenges in pain management
Min Cheol Chang
Journal of Yeungnam Medical Science.2022; 39(3): 179. CrossRef - Synucleinopathy in Amyotrophic Lateral Sclerosis: A Potential Avenue for Antisense Therapeutics?
Bradley Roberts, Frances Theunissen, Francis L. Mastaglia, P. Anthony Akkari, Loren L. Flynn
International Journal of Molecular Sciences.2022; 23(16): 9364. CrossRef - Herbal medicine and acupuncture relieved progressive bulbar palsy for more than 3 years: A case report
Siyang Peng, Weiqian Chang, Yukun Tian, Yajing Yang, Shaohong Li, Jinxia Ni, Wenzeng Zhu
Medicine.2022; 101(45): e31446. CrossRef
- Figure
- Related articles
-
- What is the disease burden from childhood and adolescent obesity?: a narrative review
- The pathophysiology of diabetic foot: a narrative review
- Management of diabetic foot ulcers: a narrative review
- Multidisciplinary approach to sarcopenia: a narrative review
- Advances in management of pediatric chronic immune thrombocytopenia: a narrative review

 E-Submission
E-Submission Yeungnam University College of Medicine
Yeungnam University College of Medicine PubReader
PubReader ePub Link
ePub Link Cite
Cite


